Robotic totally endoscopic coronary artery bypass grafting—port placements, internal mammary artery harvesting and anastomosis techniques
Introduction
Robotic total endoscopic coronary artery bypass (TECAB) has been evolving over the past two decades and currently with its technological advances serves as the only reasonable approach for endoscopic coronary surgery (1). Since the procedure’s inception in Paris in 1998 by Loulmet and colleagues, dedicated centers have advanced the procedure from single vessel left internal mammary to left anterior descending artery bypasses to multivessel revascularizations, as well as synchronous hybrid procedures in the modern era (2-5). Robotic TECAB can be performed on a beating heart using an endostabilizer when available, or on peripheral cardiopulmonary bypass with cardioplegia. The status of the procedure as well as results and trends were published in two recent review articles by the senior author (6,7). We herein describe the arrested heart version of the procedure.
Patient selection
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this article, the accompanying video and images.
All patients who qualify for elective coronary revascularization surgery can be evaluated as potential candidates for TECAB. However, in patients with cardiomegaly, acute coronary syndrome, cardiogenic shock, thoracic deformities, restrictive left pleural spaces, severely impaired lung function, morbid obesity, and severe multisystem organ dysfunction may be considered relatively contraindicated for the overall approach. Those with <8 mm femoral arteries, severe aorto-iliac atherosclerosis, and obstructed inferior vena cava are not candidates for peripheral bypass supported approaches. In all other patients who present for TECAB, the preoperative computer tomography scan of the chest provides important information for the surgeon on the location of coronary targets and a potential intramyocardial course as well as the diameter of the ascending aorta for placement of the endoaortic occlusion balloon. We use a 35 mm ascending aortic diameter as the upper limit for size. Other anatomical features such as the thickness of the pericardial fat pad and subcutaneous tissue at the nipple line have been shown to impact the duration of robotic left internal mammary artery (LIMA) harvesting, whereas LIMA-left anterior descending artery (LAD) anastomotic and overall operative times have been shown to be longer when the heart is closer to the chest wall in beating heart TECAB but not in arrested TECAB (8). These specifics, along with the availability of the endostabilizer, may guide the surgeon’s decision making on whether to arrest or perform beating heart TECAB.
Anesthesia management
Patients receive a paravertebral block on the ipsilateral thorax, which is placed by the anesthesia pain services. General anesthesia is induced, and a double lumen endotracheal tube is inserted. Alternatively, the Rusch EZ-Blocker Endobronchial Blocker (Teleflex; Morrisville, NC, USA) can be used. Monitoring consists of continuous electrocardiography, pulse oximetry, capnography, arterial blood pressure and urine output monitoring, as well as near infrared spectroscopy monitoring of the brain and both legs. Percutaneous defibrillator patches are placed on the right chest anteriorly and left chest posteriorly. In cases of endoballoon (Edwards IntracludeTM; Irvine, CA, USA) use for cardioplegic arrest, we place bilateral radial artery pressure monitoring lines that are used for monitoring the balloon position.
Surgical techniques
Patient positioning, prepping, and draping—preparation for procedure start
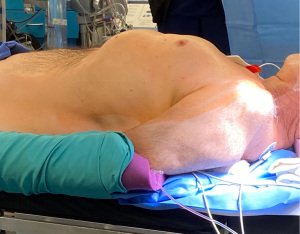
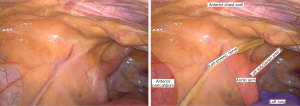
The patient is positioned supine with the left chest elevated to about 30 degrees (Figure 1), arms remain tucked. The surgical robot is draped while anesthesia is induced. The patient is prepped and draped leaving all options open for conventional coronary bypass surgery (Figure 2). The later excursions of the robotic arm towards the patient’s head have to be taken into account at this time point. Operating room lights and monitors should be positioned in a way that collisions at robot docking are avoided. For good communication between anesthesia and surgical team the curtain between the two teams should be set relatively low. After prepping and draping the patient, we make sure that all usual cables and lines, including the heart lung machine tubing, are opened on the operating table. We also confirm that the carbon dioxide gas (CO2) insufflation line, the robotic cautery cable, and the robotic camera are prepared. Then the team performs the surgical time out.
Setup of the robotic system and surgical team composition
We use a Da Vinci Xi (Sunnyvale, CA, USA) dual console surgical system, which allows for teaching and sharing of procedure components. In most instances, two attending level cardiac surgeons perform the critical parts of the procedure. A surgical resident and a midlevel surgical assistant also participate.
Port placement
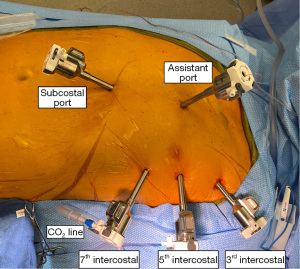
It is very important that the surgeon makes sure that the left lung is collapsed before starting the port placement. Port holes are well cauterized to prevent later bleeding. The camera port is inserted into the left 5th intercostal space on the anterior axillary line. This insertion point can also be found by placing the middle finger of the right hand on the jugulum and the middle finger of the left hand on the xiphoid angle (not the tip of the xiphoid). Where the thumbs of the surgeon meet the closest interspace is palpated and the port is inserted there. The CO2 line is connected to the port immediately to create more intrathoracic space for insertion of the other ports. The left and right instrument ports are placed into the 3rd and 7th intercostal spaces or four finger widths off the camera port (Figure 3). Ports form a very flat triangle. It is important that the surgical assistant follows the port insertion with the robotic camera. The right instrument port is essentially always visualized with the scope. For the left instrument port this may be more difficult. For ergonomic reasons and in order not to compromise camera view by CO2 flow, the CO2 line is connected to the left instrument port before robot docking.
Robot docking
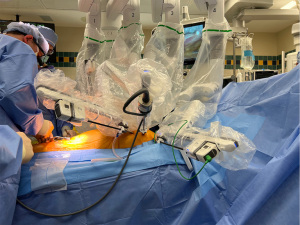
It is important to start the docking process already during port insertion so that the arms are ready for the connection immediately. This requires excellent communication with the circulator and good robot driving skills of this person. We use the laser targeting system and make sure the laser cross is right at the camera port insertion site. The following arms on the Da Vinci Xi system are used in robotic TECAB: arm 2 for the left instrument, arm 3 for the camera, arm 4 for the right instrument. Arm 1 is for the subcostal port and will be docked later. Docking is easiest if the surgeon holds the robotic arm and guides it towards the port. The assistant presses the lever and the surgeon leads the connection piece on the port to the arm. Successful docking is confirmed by an acoustic signal from the machine. The camera arm is docked first and the 30-degree camera is inserted using the “up view”. The surgical assistant then guides the camera towards the right instrument port which is docked when in view. The electrocautery spatula is inserted right away, connected to the cautery cable, and advanced into the chest towards the internal mammary artery (IMA). This requires that the outer end of the arm is lowered and the instrument takes a tangential slightly upward course. The camera is then moved leftward, and the outer end of the left arm is lowered to ensure tangential insertion of a robotic DeBakey forceps. The instrument is directed towards the IMA and the previously inserted cautery spatula. Figure 4 demonstrates the fully docked Da Vinci Xi robot for robotic TECAB.
IMA harvesting
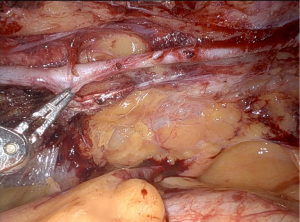
Graft harvesting is carried out with a camera 30 degrees up view. Before starting the harvesting the thoracic cavity is inspected. If necessary, lung adhesions are taken down. The main landmarks that come into sight are the pericardial sac with the pericardial fat pad, the left phrenic nerve, the hilum of left the lung, and the left subclavian artery (Figure 5). The left aortic arch can be appreciated through the mediastinal tissue.
The left IMA can commonly be seen pulsating underneath the endothoracic fascia, mostly in its upper third. This is where takedown is started. It is extremely important that before initiating harvesting, we confirm that the cautery is set at low power (level 2 on the Xi system) to avoid thermal injury. The endothoracic fascia is incised lateral to the LIMA pedicle and detached in its whole length. We usually let it suspend with gravity so that the artery and the concomitant veins come into sight. Alternatively, the fascia can be split just above the artery. This, however, requires robotic LIMA harvesting experience. At the caudal end, the endothoracic fascia meets the transverse thoracic muscle which is divided. The IMA proper is usually covered with a thin adventitial layer. If this layer is detached the artery “pops” out and harvesting becomes straight forward. We harvest the IMA in skeletonized technique because better length can be obtained and because handling of the graft is easier when it comes to anastomotic suturing. The robotic surgeon should frequently check visually for adequate pulsations of the artery.
We first expose the artery in its whole length on the sternal side. After that we mobilize it completely at the border between the proximal and middle third and work in caudal direction. The IMA adventitia can be gently grasped with robotic DeBakey forceps during the harvesting process. The surgeon makes sure to expose each side branch properly before it is divided. Smaller side branches are cauterized close to the chest wall. Larger side branches are clipped close to the LIMA and distally also cauterized near the chest wall. Very large branches are clipped twice and divided using the robotic Pott’s scissors. If a clip needs to be inserted the surgical assistant makes sure not to pull back the instrument port because repetitive re-insertions can lead to difficult chest wall bleeding. If the port (mostly the right instrument port) is pulled out, the console surgeon looks at the port, the assistant surgeon lifts up the outer end of the robotic arm and re-inserts the port perpendicular to the chest wall. It is important to never push in an instrument against resistance. This way chest wall injuries can be avoided. If a portion of the IMA is difficult to reach swapping left and right instrument can sometimes help.
The LIMA is distally mobilized until Its bifurcation is reached (Figure 6). Proximally we work towards the left subclavian vein which we sometimes see but we do not force its visualization. We also pay attention to the phrenic nerve in this area. After heparinization we look at the distal end of the LIMA gently hold it with the robotic DeBakey forceps on the right instrument arm and clip it with clips coming through the left instrument port. Two clip appliers are used so that rapid reloading is possible. Two clips are placed distally and one clip proximally. Then the vessel is divided using robotic Pott’s scissors. The LIMA is then brought cranially and dropped into the chest for auto-dilatation. We do not use any topical or intraluminal vasodilators because the vessel will nicely expand during the following steps of the procedure.
Insertion of assistance ports
Parasternal assistance port
With camera view still 30 degrees up, the console surgeon looks into the LIMA harvesting bed opposite the insertion of the camera port. The patient side assistant palpates the corresponding intercostal space next to the sternum, places a small port incision and inserts an 8 mm port under endoscopic view. It is important to place this port into the IMA bed and not into the endothoracic fascia or muscle because the latter may lead to difficult bleeding at the completion of the case.
Subcostal assistance port
Another 8 mm assistance port is placed subcostally two finger widths off the xiphoid angle and a little caudal to the left costal margin into the left pleural cavity. The port is inserted by the patient side assistant who again follows the insertion maneuvers on the video monitor. This port is directed towards the patient’s left shoulder. The console surgeon helps from inside by resecting fat tissue in the xiphoid region. The subcostal port is then docked to arm 1 on the Da Vinci Xi robot. Assisting instruments such as the long tip forceps can be inserted through this port.
For these ports, starting with a dilating maneuver with a tonsil clamp is recommended. Surgical material necessary for the next steps can be brought in through both ports using an endoscopic grasping instrument.
Rationale for using cardiopulmonary bypass and cardioplegia for robotic TECAB
Robotic TECAB surgery has been described on the beating heart and on the arrested heart in the literature and has been carried out by the senior author of this paper in both versions (5-7,9,10). Currently the robotic target vessel endostabilizer for the beating heart TECAB is not produced by the company Intuitive (Sunnyvale, CA, USA) which offers the Xi robotic system. Therefore, cardioplegic arrest is the only way to carry out a robotic TECAB operation at the current time. In addition, results in the literature show that with proper patient selection the arrested heart, TECAB can be carried out with excellent results that are at least as good as those in beating heart TECAB (3,6,7,11). In addition, recent reports on minimally invasive coronary artery bypass grafting (CABG) carried out on cardiopulmonary bypass with cardioplegic arrest demonstrate safe conduct and a high grade of complete revascularization (1,12). Robotic endoscopic sewing of an anastomosis under cardioplegia is technically easier than on the beating heart and arresting the heart offers more space inside the chest as both lungs can be deflated. The heart is unloaded with this approach and can be mobilized for positioning very well, which helps with exposure of the target vessels on the lateral wall and back wall of the heart. Lastly the surgeon can take full advantage of the tremor free work that the surgical robot offers. Alternatively, Balkhy et al. (13) have described the conduct of multivessel TECAB using the C. Port Flex A distal anastomosic device (Cardica; Redwood City, CA, USA) to automate the distal anastomoses in beating heart TECAB. This group demonstrated a 94% anastomotic patency with this technique at 4-month angiographic follow-up. But this product is currently not available on the market.
Cannulation for cardiopulmonary bypass and induction of cardioplegia
The femoral artery and vein are exposed using a small, oblique 4 cm incision in the left groin. Heparin is given. Only the anterior wall of these vessels is dissected. Going around the vessels increases the chances of a lymphatic leak and should be avoided. We place a wired 6 Fr wire reinforced distal perfusion catheter (Super Sarrow-Flex PSI Set, Arrow) in all cases because pump times may be long, specifically in multivessel TECAB. A 5-0 Prolene purse string suture is placed on the proximal superficial femoral artery and the distal perfusion catheter is inserted and flushed with heparin saline solution. We strongly recommend the use of a micro-puncture set before insertion of the distal perfusion catheter in Seldinger technique.
Then 4-0 Prolene purse string sutures are placed on the femoral artery and vein. First the multistage venous cannula (Biomedicus, Medtronic, Minneapolis, MN, USA) is inserted under trans esophageal echocardiography (TEE) guidance. It is important that the surgeon has a good view of the TEE him or herself to place the cannulae properly. The anesthesiologist presents the superior vena cava and the and the surgeon advances the guidewire through the puncture needle. With proper view, the “J” end of the guidewire can be appreciated as it moves up the superior vena cava (SVC). Only with the guidewire clearly located in the SVC should the surgeon proceed. The femoral vein is then serially dilated, and the cannula is moved up into the SVC. Do not pull back the obturator early but make sure you see the double contour of the cannula moving into the SVC before you pull it back. This way dislocations of the cannula tip into the interatrial septum or into the right ventricle can be avoided. The cannula can be flushed with heparin saline, but only if no patent foramen ovale is present. It is connected to the venous line of the heart lung machine tubing.
The arterial cannula with the side arm for the endoballoon (Edwards EndoReturn) is then inserted into the femoral artery after advancing a guidewire into the descending thoracic aorta (Figure 7). Make sure that the valve on the side arm is closed before insertion. Also advance the cannula into the artery as far as possible so that the endoballoon reaches the proximal ascending aorta in tall patients. After securing the cannula to the tourniquet the obturator is removed and the cannula clamped. It is then connected to the arterial line of the heart lung machine tubing. We prefer the 23 Fr cannula over the 21 Fr cannula because the latter may cause high line pressures with the balloon catheter in place. The distal perfusion cannula is connected as well. All cannulae are secured with tourniquets and additionally fixed to the skin with silk sutures.
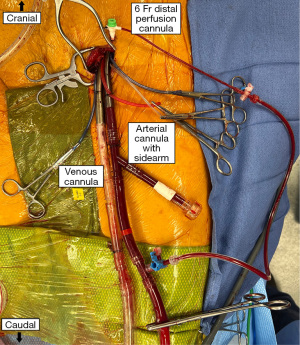
The endoballoon catheter (IntraClude device) has been prepared by the scrub tech before beginning of the case. The de-aired and flushed catheter is inserted into the side arm of the arterial perfusion cannula and pushed forward into the cannula proper. Then the guidewire is advanced into the descending thoracic aorta and into the ascending aorta under TEE vision. Again, it is of utmost importance that the surgeon handling the catheter has an excellent view of the TEE monitors him or herself. Once the position of the guidewire above the aortic valve is confirmed the balloon is brought up and placed right above the aortic valve. Then the guidewire is pulled back and the valve at the catheter end is closed to prevent aspiration of air. The pressure lines for aortic root pressure and balloon pressure are connected to the corresponding pressure transducers and flushed. The Y-piece for cardioplegia and vent is also connected to the balloon catheter and the cardioplegia and vent lines.
General principles of wire and catheter handling apply for installation of remote cardiopulmonary bypass (CPB) and the endoballoon. No component should ever be advanced without adequate visualization or against resistance. Aspiration of air into the system should be avoided. If the guidewire for the endoballoon cannot be visualized in the ascending aorta the catheter can be advanced into the distal aortic arch and a new guidewire positioning maneuver can follow.
If two experienced surgeons are available, the IMA harvesting and cannulation can be carried out in parallel with an initial activated clotting time (ACT) of 300 seconds. Before going on bypass full dose heparin is given.
Start of cardiopulmonary bypass and induction of cardioplegia
After confirming adequate ACT levels, cardiopulmonary bypass is started slowly. All team members should look at the descending thoracic aorta at the start of CPB. This way a retrograde dissection can be detected immediately. Increases in arterial line pressure need to be reported immediately for the same reason. Once no ejections of the heart are seen, the aortic endoballon can be inflated. Before inflation, cardioplegia is flushed into the vent line and adenosine syringes are prepared. Under TEE view the balloon is inflated. The surgeon who injects can feel as the balloon touches the ascending aorta and injects until a good seating of the balloon can be noted. Slack of the catheter is pulled back and the catheter is secured at the perfusion cannula side arm with a locking piece. This maneuver is important as catheter slack can lead to inadequate seating of the balloon and mobilization of plaque or wall injury on the aortic arch. Good balloon position is confirmed again, then adenosine (6 mg diluted to 20 mL of normal saline) is injected into the aortic root, which is seen as a “snow storm like” picture. Asystole is induced and cardioplegia infusion starts. Should electrokardiogram (EKG) activity still be noted adenosine can intermittently be injected as needed. Balloon pressure can be adjusted if migration occurs or if cardioplegia flow or venting is inadequate. Balloon migrations can be detected directly on TEE or by loss of the right radial arterial line pressure. Sometimes complete deflation of the balloon and repositioning is necessary. Balloon migrations into the left ventricle can also happen. This is probably harmless in normal aortic valves but may cause damage to the leaflets if they are thickened or calcified. Systemic cooling is applied depending on the cardiopulmonary bypass and cardioplegia times that are expected.
Creation of a pericardial drainage hole
The following steps are performed with robotic scope view down. We cut a small drainage hole into the pericardium posterior to the phrenic nerve. This ensures that accumulations of blood in the pericardial sac are avoided during the further conduct of the procedure.
Pericardial fat pad removal and pericardiotomy
The pericardial fat pad is then removed. We usually use a robotic long-tip forceps to grab the fat pad because it may be large and slippery and then mobilize it with electrocautery at level 3 or 4. We start at the upper sternal portion and move caudally and laterally, so that the anterolateral surface of the pericardial sac is nicely exposed. Then the pericardium is opened using the same instruments starting above the right ventricular outflow tract and moving in the direction of the sternum and caudally to the reflection of the pericardium. The incision is then carried laterally. Proximally we move towards the left atrial appendage and stop 2 cm above the phrenic nerve.
It is important to keep enough pericardial sac so that the left ventricle does not herniate out of it. Fat pad removal and pericardiotomy can be performed before going on pump but is usually much faster on pump and under cardioplegia.
Robotic coronary anastomosis
In the TECAB procedure the anastomosis is carried out using the robotic instruments. This is in contrast to the minimally invasive direct coronary artery bypass (MIDCAB), in which the robot is undocked after pericardiotomy, and the procedure continues through a mini-thoracotomy using handheld surgical instruments.
We first identify the target vessel, which is the LAD in essentially all cases. It is important to see this vessel running towards and around the apex of the heart. It must not be confused with diagonal branches. In many cases, the LAD is easily identifiable and accessible. If not, a long-tip forceps can be brought through the subcostal port and used for better exposure gently grabbing the epicardium.
We then cut the epicardium above the vessel using robotic Pott’s scissors and make the final decision on the landing zone of the LIMA. The graft is then placed beside the LAD and clamped with a bulldog clamp that is handed over to the console surgeon through the parasternal assistance port. Using Pott’s scissors, the distal clip is cut off the graft and the vessel is incised for a beveled anastomosis.
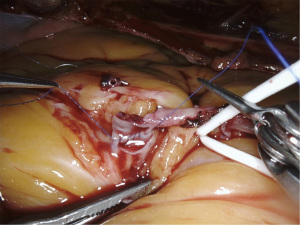
For incision of the target vessel, we have developed a workaround as the old robotic snap-fit instrument, which was available on older generations of the Da Vinci, that holds a special sharp lancet beaver knife is not in production for the Xi system. A regular lancet (Stab Knife, 15 degrees 3.0 mm Blade, Sharpoint Surgical Specialties), is connected to a piece of 14 Fr red rubber catheter and brought into the chest (Figure 8). It can be held using long-tip forceps and used for incision of the target vessel. The incision is extended as needed using robotic Pott’s scissors. Then a 7 cm long double armed 7-0 Pronova suture (Ethicon Inc., Somerville, NJ, USA) is handed over to the console surgery through the parasternal assistance port and anastomotic suturing starts using two micro forceps. For details see the video (Video 1).

In brief, the posterior wall is sutured first using graft inside-out target vessel outside in stitches (Figure 9). We then go around the heel and finally work around the toe going outside in on the graft and inside out on the target vessel. We have tried other versions avoiding outside-to-inside stitches on the target vessel but ergonomically our original approach proved to be the best. The target vessel’s toe and heel can be gently probed with the micro-forceps before tying off the suture. As there is no assistant controlling suture tension the robotic surgeon pulls frequently on both suture ends to avoid slings. Frequent inspection for slings is important. Slings can be pulled up with the suture needle. If there is too much backflow from the target vessel, we make sure that the venous drainage and root venting function properly and that the endoaortic balloon pressure is adequate. If all of this is appropriate, we use a silastic tape to occlude the proximal target vessel.
Following completion of the anastomosis, the bulldog on the LIMA is then opened to check for leakages. The latter can be controlled with single 7-0 Pronova sutures with excellent overview as the operative field is significantly magnified. We cannot stress enough the importance of multiple robotic suturing exercises in virtual, dry-lab, and wet-lab models before performing a robotic coronary anastomosis in the clinical setting. Triple digit numbers of simulations are recommended. With proper training, however, the anastomosis can be carried out with a high comfort and safety level.
Myocardial reperfusion and weaning from CPB
Before opening the endoaortic balloon we make sure all foreign material is removed from the operative field as the heart will often become hyperdynamic and removal might be very difficult. Prior to opening the endoballoon, it is important to actively vent the root again because some air may have accumulated. The balloon is deflated and in single vessel TECAB the heart usually starts beating in sinus rhythm spontaneously. Should the heart fibrillate, one option before external fibrillation is to inflate the endoballoon again and to give adenosine and cardioplegia. After balloon deflation, sinus rhythm usually comes back.
During reperfusion, graft flow measurements are carried out using a 3 mm transit time flow probe without a handle brought in through the subcostal port. The operative field is thoroughly checked for bleeding and hemostasis is achieved. It is important to keep a DeBakey forceps and cautery in the IMA bed during the weaning process because later insertion of these instruments can be difficult.
We come off cardiopulmonary bypass using two lung ventilation and reduction of CO2 pressure to 2 mmHg. Some drop in oxygenation is still sometimes present after weaning but is typically very transient. Having confirmed adequate left ventricular (LV) function and absence of myocardial ischemia, the patient is decannulated and protamine is given. We make sure good pulsations of the proximal superficial femoral artery are present after decannulation.
Concluding steps
We then deflate the left lung again, insufflate CO2 at 8 mmHg and inspect the operative field for presence of clot and for residual bleeding sites which are controlled. Blood accumulations in the left pleural space are evacuated with a suction tube. Then the robotic instruments are removed, the robotic arms are undocked, and the machine is moved away from the operating table.
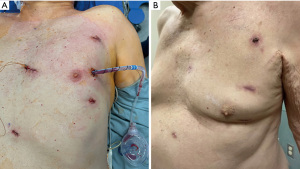
It is of utmost importance to keep the ports in place and inspect all portholes with the robotic camera from inside. They are cauterized as necessary and packed with Surgicel (Ethicon Inc.). A Blake drain is inserted through the camera porthole under videoscopic vision. Incisions are infiltrated with local anesthetic and closed in layers (Figure 10). Extubation in the operating room is common (11).
Comments
Main advantages of arrested heart TECAB
Using the heart lung machine offers the advantage that both lungs can be deflated and intrathoracic space can be gained. Unloading of the heart is another factor that contributes to this gain of space. Vision and surgical flexibility increase dramatically. Anastomotic suturing is easier than on the beating heart and the flaccid arrested heart allows for better exposure of target vessels. The surgeon can take full advantage of tremor free work with the surgical robot.
Challenges
The main challenge with this procedure is that remote access heart lung machine perfusion and application of the endoballoon require an additional learning curve. We highly recommend that surgeons and robotic teams acquire the endoballoon skill set in procedures other than TECAB before they use it in endoscopic coronary bypass surgery. Contraindications described in our paper need to be taken very seriously, specifically the contraindications for use of remote access cardiopulmonary bypass. Pump times and myocardial ischemic times in TECAB are longer than in equivalent CABG procedures through sternotomy and only patients who can tolerate these prolonged perfusion times and cardioplegic arrest times should be chosen.
In the United States and under Food and Drug Administration regulations, the TECAB procedure is currently being performed on the beating heart using the Da Vinci Si robotic system for which the robotic endostabilizer is still available. The arrested heart version of the TECAB procedure can be carried out under the same regulations using both the Da Vinci Si and Xi systems. In Europe, however, recent new medical device regulations (MDR) have restricted the application of the Intuitive Da Vinci Si and Xi systems to harvesting of the IMA only.
Conclusions
With proper patient selection and proper stepwise training including simulation robotic TECAB in the arrested heart technique is a reproducible and safe procedure which can be performed with a high comfort level.
Acknowledgments
We thank the multidisciplinary teams at UPMC Presbyterian and UPMC Passavant Hospitals for all their contributions in establishing the robotic cardiac surgery program.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Johan van der Merwe and Filip P. Casselman) for the series “International Perspectives on Minimally Invasive Coronary Artery Revascularization” published in Journal of Visualized Surgery. The article has undergone external peer review.
Peer Review File: Available at https://jovs.amegroups.com/article/view/10.21037/jovs-22-10/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure forms (available at https://jovs.amegroups.com/article/view/10.21037/jovs-22-10/coif). The series “International Perspectives on Minimally Invasive Coronary Artery Revascularization” was commissioned by the editorial office without any funding or sponsorship. IS serves as an unpaid editorial board member of Journal of Visualized Surgery from August 2019 to July 2023. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images and videos.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pettinari M, Gianoli M, Palmen M, et al. Robotic coronary revascularization in Europe, state of art and future of EACTS-endorsed Robotic Cardiothoracic Surgery Taskforce. Interact Cardiovasc Thorac Surg 2022;35:ivac108. [Crossref] [PubMed]
- Loulmet D, Carpentier A. Endoscopic coronary artery bypass grafting with the aid of robotic assisted instruments. J Thorac Cardiovasc Surg 1999;118:4-10. [Crossref] [PubMed]
- de Cannière D, Wimmer-Greinecker G, Cichon R, et al. Feasibility, safety, and efficacy of totally endoscopic coronary artery bypass grafting: multicenter European experience. J Thorac Cardiovasc Surg 2007;134:710-6. [Crossref] [PubMed]
- Bonaros N, Schachner T, Lehr E, et al. Five hundred cases of robotic totally endoscopic coronary artery bypass grafting: predictors of success and safety. Ann Thorac Surg 2013;95:803-12. [Crossref] [PubMed]
- Bonatti JO, Zimrin D, Lehr EJ, et al. Hybrid coronary revascularization using robotic totally endoscopic surgery: perioperative outcomes and 5-year results. Ann Thorac Surg 2012;94:1920-6; discussion 1926. [Crossref] [PubMed]
- Bonatti J, Wallner S, Winkler B, et al. Robotic totally endoscopic coronary artery bypass grafting: current status and future prospects. Expert Rev Med Devices 2020;17:33-40. [Crossref] [PubMed]
- Bonatti J, Wallner S, Crailsheim I, et al. Minimally invasive and robotic coronary artery bypass grafting-a 25-year review. J Thorac Dis 2021;13:1922-44. [Crossref] [PubMed]
- Wehman B, Lehr EJ, Lahiji K, et al. Patient anatomy predicts operative time in robotic totally endoscopic coronary artery bypass surgery. Interact Cardiovasc Thorac Surg 2014;19:572-6. [Crossref] [PubMed]
- Bonatti J, Schachner T, Bernecker O, et al. Robotic totally endoscopic coronary artery bypass: program development and learning curve issues. J Thorac Cardiovasc Surg 2004;127:504-10. [Crossref] [PubMed]
- Stastny L, Kofler M, Dumfarth J, et al. Long-Term Clinical and Computed Tomography Angiographic Follow-up After Totally Endoscopic Coronary Artery Bypass Grafting. Innovations (Phila) 2018;13:5-10. [Crossref] [PubMed]
- Göbölös L, Ramahi J, Obeso A, et al. Robotic Totally Endoscopic Coronary Artery Bypass Grafting: Systematic Review of Clinical Outcomes from the Past two Decades. Innovations (Phila) 2019;14:5-16. [Crossref] [PubMed]
- Babliak O, Demianenko V, Melnyk Y, et al. Complete Coronary Revascularization via Left Anterior Thoracotomy. Innovations (Phila) 2019;14:330-41. [Crossref] [PubMed]
- Balkhy HH, Wann LS, Krienbring D, et al. Integrating coronary anastomotic connectors and robotics toward a totally endoscopic beating heart approach: review of 120 cases. Ann Thorac Surg 2011;92:821-7. [Crossref] [PubMed]
Cite this article as: Seese L, Ashraf SF, Davila A, Coyan G, Joubert K, Zhang D, Kaczorowski D, West D, Sultan I, Bonatti J. Robotic totally endoscopic coronary artery bypass grafting—port placements, internal mammary artery harvesting and anastomosis techniques. J Vis Surg 2023;9:4.