Uniportal subxiphoid video-assisted thoracoscopic anatomical segmentectomies—two case reports
Introduction
Minimally invasive thoracic surgical techniques are proved significantly improve enhanced early post-operative recovery. Open techniques have been associated with increased morbidity and post-operative pain especially in major lung resections. Intercostal nerve injury has been associated with early morbidity with acute pain but also with long term effects due to chronic pain. Video-assisted thoracoscopic surgery (VATS) is a minimally invasive technique which was thought to have an immediate impact on the incidents of chronic pain after thoracic surgery. However, chronic pain rates were not different when compared to open thoracotomy techniques (1). This was believed to be due to the camera manoeuvring through the intercostal port which is still a confined space where a crush nerve injury cannot be avoided. With the inception of uniportal VATS, incidents of chronic nerve injury pain were reduced but post-operative pain remained a persistent issue (2).
Lately, uniportal subxiphoid VATS (SVATS) has been widely described in the literature and is recently used for a variety of thoracic surgeries such as lobectomies, segmentectomies, bullectomies and thymectomies. SVATS technique includes a single 4 cm vertical incision which spares the muscles and it is performed in the subxiphoid area, allowing the resection to be carried out without any irritation to the intercostal bundle. Subsequently, this is minimising the post-operative pain for both chronic and acute pain issues, compared with the standard intercostal VATS technique. This is due to the location of the surgical approach as the surgeon is able to remove the specimen through the pleural cavity without spreading any ribs and eliminating the incidence of intercostal nerve injury (3).
One of the indications for SVATS segmentectomy is lung cancer at early stage, with no involvement of the mediastinal lymph nodes. However, SVATS can be also offered when the patients are not candidates for lobar resection due to previous operations or history of interstitial lung disease. Here we describe a challenging case of a uniportal right SVATS S1 segmentectomy on a patient with a previous history of pulmonary fibrosis and a case of uniportal left upper lobe SVATS trisegmentectomy representative of our department’s experience of SVATS segmentectomies. We present this case in accordance with the CARE reporting checklist (available at https://jovs.amegroups.com/article/view/10.21037/jovs-21-53/rc).
Case description
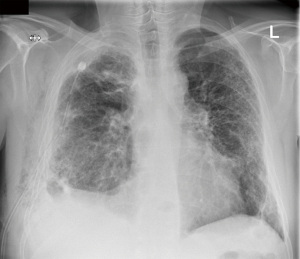
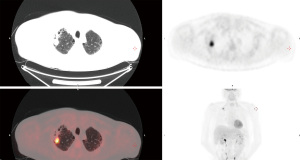
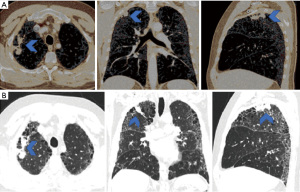
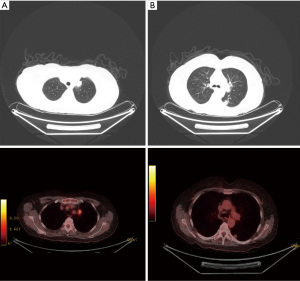
A 59-year-old male was referred through a multidisciplinary team (MDT) meeting, for a right upper lobe tumour resection. Positron emission tomography computed tomography (PET-CT) demonstrated an increasing 26×18 mm tumour in the right upper lobe (apical segment) with an SUVmax 10.4 with involvement of the visceral pleura (Figure 1). This was previously biopsied and diagnosed as adenocarcinoma. Additionally, there was a previously known stable 28 mm soft tissue lesion with an SUVmax 3.8, which was diagnosed as an aspergilloma. A 3D imaging reconstruction was performed preoperatively for surgical planning (Figure 2). No significant mediastinal or hilar nodes were noticed.
Comorbidities included rheumatoid associated interstitial fibrosis on methotrexate and rituximab, rheumatoid arthritis and previous respiratory infection with Haemophilus influenza requiring intravenous antibiotics. He was also an ex-smoker with a history of 25 pack years. The American Society of Anaesthesiologists (ASA) grade was two and the Eastern Cooperative Oncology Group (ECOG) performance status was one.
We also performed lung function tests (LFTs) which reported forced expiratory volume (FEV1) of 2.98/83% predicted, forced vital capacity (FVC) of 4.55/98% and gas transfer factor of 63%. The patient was discussed in multiple MDT meetings and after taking into consideration his underlying pulmonary fibrosis, a radiotherapy option was discounted. Therefore, a surgical resection with a view to an anatomical sub lobar resection thought to be the most appropriate treatment option.
The operation was performed with general anaesthesia and an endotracheal double lumen tube was used to allow selective lung ventilation. The patient was positioned in the left lateral decubitus position and both subcostal transversus abdominis and paravertebral blocks were performed using 0.25% of levobupivacaine.
In the in the sternocostal angle, we performed a 4 cm vertical incision and divided the linea alba. In order to enter the pleural cavity, we used blunt dissection and we positioned an Alexis (Applied Medical, CA, USA). We used a 10 mm thirty degree camera and custom made subxiphoid VATS 42 m instruments were specifically ordered and designed for this approach.
The first step was to identify any adhesions or unexpected pathology during the inspection of the pleural cavity. This was followed by an infiltration of right phrenic and vagus nerves with 0.25% levobupivacaine local anaesthetic. We dissected the mediastinal pleura, the fissure before we started with the dissection of the vessels and the bronchus for the S1 apical right upper lobe segment using endoscopic staplers. Finally we performed a systematic mediastinal lymph node sampling. The removal of the specimen was through an endoscopic retrieval bag.
The patient was extubated in theatre and was admitted to the intensive care unit for observation. During the first night an intravenous patient controlled analgesia was utilised but was ceased the next day as the patient did not use it anymore. Pain relief was continued with paracetamol and oral morphine as needed. Day 1 post operatively we started with our enhanced rehabilitation program and the patient was transferred to the ward. His postoperative care was complicated by persistent air leak, such that he was discharged home with a portable chest drain in situ (Figure 3). Histology demonstrated a solid poorly differentiated adenocarcinoma pT3 N0 (PL3, R0) and an aspergilloma.
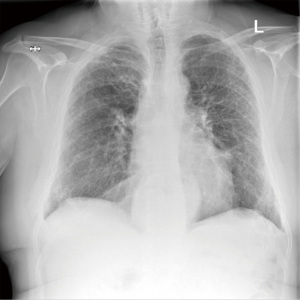
After an accidental chest drain removal, the patient was readmitted with worsening surgical emphysema and right-sided pneumothorax. A new 28 Fr chest drain was immediately inserted. After weekly reviews by the surgical team and the lung defence team, the patient was treated with prophylactic oral antibiotics and multiple blood pleurodesis for his persistent air leak. Two months after the initial operation the air leak stopped and the chest drain was removed with no further complications (Figure 4).
On a 6-month follow-up, the patient reported worsening shortness of breath and joint pain. Follow-up respiratory tests showed an FEV1 of 69% predicted and FVC of 89%. Gas transfer factor was unable to be measured. The follow-up CT scan showed no change in the pulmonary fibrosis and there was no evidence of local or distant disease recurrence.
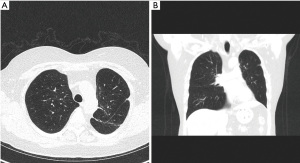
A 62-year-old-female was referred through a MDT meeting, for a left upper lobe tumour resection. The PET-CT demonstrated an increasing 28×18 mm tumour in the left upper lobe with an SUVmax 11.2 (Figure 5). This was previously biopsied and diagnosed as adenocarcinoma. No significant mediastinal or hilar nodes were noticed. There was also a 17×15 mm nodule in the apical segment of the left lower lobe with unknown aetiology (Figure 5).
Comorbidities included COPD, epilepsy, adrenal insufficiency, fibromyalgia and giant cell arteritis. ASA grade was 2 and ECOG performance status was 0.
Respiratory function tests showed FEV1 of 1 L/46% predicted, FVC of 2.29 L/87% and gas transfer factor of 69%. After multiple MDT meetings and considering her poor LFTs, a surgical resection was suggested with a view to an anatomical sub lobar resection.
Similarly to the previous case, general anaesthesia was used for this operation. Briefly, the patient was positioned in the right lateral decubitus position. A similar incision was performed and the linea alba was dissected. After inspection of the pleural cavity, there were no adhesions reported. The left upper trisegmentectomy was performed with dissection and mobilisation of the vessels, the fissure and the bronchus, sparing the lingular segment. A systematic mediastinal lymph node sample staging was performed and the specimen was retrieved using a retrieval endoscopic bag (Video 1).
The patient was extubated in theatre and transferred back to the postoperative care ward. Medications for pain relief included oral paracetamol and codeine. Her postoperative care was also complicated by persistent air leak, such that she was discharged home with a portable chest drain in situ. Histology demonstrated a solid left upper adenocarcinoma pT2a N0 (PL1, R0) and left lower lobe wedge for adenocarcinoma pT1c N0 (PL0, R0). The patient returned to baseline performance status in D2 and was discharged in D5.
The follow-up imaging in 12 months, showed no evidence of local or distant disease recurrence (Figure 6).
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from each patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
It is well established that post-operative factors reported for procedures with a thoracoscopic approach, such as minimised post-operative pain, earlier return to pre-operative performance status and shorter length of hospital stay, are improved when compared to open techniques.
In this article we illustrate the fact that uniportal SVATS approach can be effectively and safely utilized for lung segment resection. However, from our experience, a few posterior segmentectomies could be challenging and with further surgical experience, and increased confidence, these can also be resected utilizing the SVATS technique. However, emphasis needs to be given to the fact that it is vital for the surgeons who are considering SVATS approach, need to be already familiar in standard uniportal VATS approach.
Difference in outcomes between lobectomies versus segmentectomies remains still the gold-standard for non-small cell lung cancer (NSCLC) resections. However, there is a growing debate to this with recent studies suggesting that segmentectomies could have an equivalent outcome for resection of early NSCLC tumours (4). Ginsberg et al. (5), reported an inferior recurrence rate in the sub-lobar resection group and superior long-term survival outcomes for the lobectomy resection group. Recently, there have been many studies supporting segmentectomies for early-stage lung cancer. For example, Landreneau et al. (6), identified that there is no statistically remarkable difference when compared survival and recurrence of the disease factors. A recent study suggested that patients with NSCLC tumours smaller than 2 cm, the overall survival was equivalent between lobectomy and anatomical segmentectomy (7). Additionally, preliminary results from an ongoing (phase III) randomised control study (8) by Asamura et al., comparing segmentectomy resections with lobectomy resections for NSCLC tumours less than 2 cm, has reported that segmentectomies have a 5% survival advantage over lobectomies. Preservation of pulmonary tissue is the main benefit of segmentectomy resection compared to lobectomy resections and subsequently the preservation of lung function, which has a tremendous impact on patients’ quality of life (9). As such, and specifically for patients with multiple comorbidities and poor lung function, consideration of segmentectomy resection has remained a more suitable treatment option when a lobectomy cannot be performed (10,11).
The uniportal SVATS technique avoids any intercostal incision or instrumentation. Moreover, postoperatively there are no intercostal chest drains entering the thoracic cavity and subsequently there is no impact on the intercostal neurovascular bundle when the patients are mobilising. The main benefit of this is that it allows early enhanced recovery, secretions clearance with lower respiratory tract infection events, lower incidence of venous thromboembolism episodes, and as a result a reduced hospital stay (12). More additional benefits in terms of post-operative pain include immediate post-operative but also reduced rates of chronic nerve pain.
Following our growing experience with SVATS technique for lobectomy resections, the move to SVATS segmentectomy resections was without additional complications. Experienced surgeons who are already experienced in uniportal VATS can adapt to the SVATS approach more easily. Practical differences that need to be considered are changing in the positioning of the instrument and the camera view which will improve with practice, but at that stage, the necessary practical skills can be obtained over a short learning curve (13,14). One the most important advantages of this technique is the different angle required for stapling, which can be occasionally challenging through the standard uniportal VATS approach.
Increased difficulty could be hidden in certain occasions such as left-sided procedures, patients with existing heart related disease, patients with high BMI that might affect the position of the heart and the diaphragm during the operation. Another common difficulty for segmentectomies, would be the posterior segments (S2, S6, S9 and S10), which can be challenging. Although these segmentectomies can be approachable by opening the fissure, in our experience we believe that in some cases a traditional intercostal VATS technique could be preferable and should be attempted once confidence and experience in the more accessible segments have been achieved. Finally, completing an intact lymph node dissection could be also challenging, which should only be considered once the surgeon has gained experience in this approach (15).
Conclusions
Uniportal SVATS innovative approach is becoming increasingly popular. Current studies suggest that this approach has the benefit of minimizing the duration and amount of postoperative pain, and as a result enhanced recovery program could be facilitated with an overall improvement in our patients’ care. These two reported case reports with SVATS segmentectomy approach, are representative of our department’s experience.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Michel Gonzalez) for the series “VATS Segmentectomy” published in Journal of Visualized Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jovs.amegroups.com/article/view/10.21037/jovs-21-53/rc
Peer Review File: Available at https://jovs.amegroups.com/article/view/10.21037/jovs-21-53/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.com/article/view/10.21037/jovs-21-53/coif). The series “VATS Segmentectomy” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from each patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Landreneau RJ, Mack MJ, Hazelrigg SR, et al. Prevalence of chronic pain after pulmonary resection by thoracotomy or video-assisted thoracic surgery. J Thorac Cardiovasc Surg 1994;107:1079-85; discussion 1085-6. [Crossref] [PubMed]
- Hirai K, Takeuchi S, Usuda J. Single-incision thoracoscopic surgery and conventional video-assisted thoracoscopic surgery: a retrospective comparative study of perioperative clinical outcomes†. Eur J Cardiothorac Surg 2016;49:i37-41. [PubMed]
- Liu CC, Wang BY, Shih CS, et al. Subxyphoid single-incision thoracoscopic pulmonary metastasectomy. Thorac Cancer 2015;6:230-2. [Crossref] [PubMed]
- Hwang Y, Kang CH, Kim HS, et al. Comparison of thoracoscopic segmentectomy and thoracoscopic lobectomy on the patients with non-small cell lung cancer: a propensity score matching study. Eur J Cardiothorac Surg 2015;48:273-8. [Crossref] [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Landreneau RJ, Normolle DP, Christie NA, et al. Recurrence and survival outcomes after anatomic segmentectomy versus lobectomy for clinical stage I non-small-cell lung cancer: a propensity-matched analysis. J Clin Oncol 2014;32:2449-55. [Crossref] [PubMed]
- Bao F, Ye P, Yang Y, et al. Segmentectomy or lobectomy for early stage lung cancer: a meta-analysis. Eur J Cardiothorac Surg 2014;46:1-7. [Crossref] [PubMed]
- Shi Y, Wu S, Ma S, et al. Comparison Between Wedge Resection and Lobectomy/Segmentectomy for Early-Stage Non-small Cell Lung Cancer: A Bayesian Meta-analysis and Systematic Review. Ann Surg Oncol 2022;29:1868-79. [Crossref] [PubMed]
- Nomori H, Shiraishi A, Cong Y, et al. Differences in postoperative changes in pulmonary functions following segmentectomy compared with lobectomy. Eur J Cardiothorac Surg 2018;53:640-7. [Crossref] [PubMed]
- Ali JM, Kaul P, Jiang L, et al. Subxiphoid pneumonectomy: the new frontier? J Thorac Dis 2018;10:4464-71. [Crossref] [PubMed]
- Joo S, Kim DK, Sim HJ, et al. Clinical results of sublobar resection versus lobectomy or more extensive resection for lung cancer patients with idiopathic pulmonary fibrosis. J Thorac Dis 2016;8:977-84. [Crossref] [PubMed]
- Hernandez-Arenas LA, Lin L, Yang Y, et al. Initial experience in uniportal subxiphoid video-assisted thoracoscopic surgery for major lung resections. Eur J Cardiothorac Surg 2016;50:1060-6. [Crossref] [PubMed]
- Aresu G, Wu L, Lin L, et al. The Shanghai Pulmonary Hospital subxiphoid approach for lobectomies. J Vis Surg 2016;2:135. [Crossref] [PubMed]
- Aresu G, Jiang L, Bertolaccini L. Subxiphoid video-assisted major lung resections: the Believers’ speech. J Thorac Dis 2017;9:E387-9. [Crossref] [PubMed]
- Aresu G, Weaver H, Wu L, et al. The Shanghai Pulmonary Hospital uniportal subxiphoid approach for lung segmentectomies. J Vis Surg 2016;2:172. [Crossref] [PubMed]
Cite this article as: Nizami M, Talukder S, Tweed K, Peryt A, Coonar A, Aresu G. Uniportal subxiphoid video-assisted thoracoscopic anatomical segmentectomies—two case reports. J Vis Surg 2023;9:26.