
Direct open aortic cannulation under temporary retrograde cerebral flush perfusion in acute aortic dissection Stanford type A—a case report
Introduction
The arterial cannulation strategy is an integral part of the surgical management of acute aortic dissection Stanford type A (AADA) (1,2). Cannulation of the right subclavian artery (RSA) remains the most commonly used cannulation site in aortic dissections and elective aortic arch surgery (1-3). Femoral arterial cannulation is still a regularly used strategy in many centers in acute cases (1), despite its well established limitations, especially with regards to neurological complications (2,4,5). Central arterial cannulation has been suggested as a safe, effective and fast alternative method (2,6,7). However, it is not considered a universal approach, since anatomical conditions may hinder identification of the true lumen for direct access or increase the risk for embolic complications (e.g., thrombosed false lumen, intramural hematoma) (2). Direct “open” cannulation techniques after transection of the aorta in a short period of normothermic circulatory arrest have been proposed to overcome the issue of targeting the true lumen (8-10). However, opening the ascending aorta in normothermic circulatory arrest to cannulate the true lumen directly may result in neurologic complications due to cerebral hypoperfusion or cerebrovascular emboli from air or debris. We therefore suggest combining the direct open cannulation technique with a temporary period of hypothermic retrograde cerebral perfusion (RCP) via the superior vena cava (SVC). The latter has already been described as a temporary adjunct to standard bilateral antegrade cerebral perfusion (SACP) in aortic arch surgery (11) but to our knowledge has not been described together with direct open cannulation strategies. Herein we describe an AADA case, in which direct open aortic cannulation was achieved under cerebral protection with temporary retrograde “flush” perfusion (flush RCP). Both the aortic cannulation and RCP techniques were modified in comparison to the techniques described before (8-11). We present this case in accordance with the CARE reporting checklist (available at https://jovs.amegroups.com/article/view/10.21037/jovs-21-4/rc).
Case presentation
All procedures performed in the case were in accordance with the standards of the institutional ethics committee and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
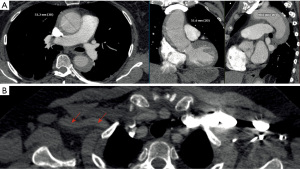
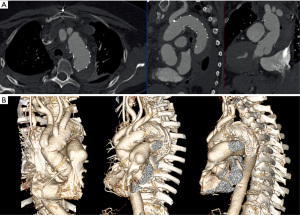
A 67-year-old female patient presented on November 4th, 2019 at a regional hospital with sudden backpain and collapse. The patient’s medical history showed arterial hypertension, hyperlipidemia and smoking as cardiovascular risk factors. She had suffered from pulmonary embolism two years before. At the time of presentation, she was not anticoagulated. Blood chemistry showed elevated D-Dimers. The patient underwent immediate computed tomography angiography (CTA) to rule out pulmonary embolism. CTA showed an AADA as an intramural hematoma of the entire aorta from the aortic root to the infrarenal aorta. The ascending aorta was aneurysmatic (52 mm), and the proximal descending aorta showed a diameter of 41 mm (Figure 1A). The RSA was dissected (Figure 1B). The patient suffered from persisting backpain despite sufficient blood pressure control and morphine treatment. The patient was referred to our tertiary center with the initial diagnosis of acute aortic dissection Stanford type B. On arrival of the patient and after evaluation of the external CTA scans the correct diagnosis of an AADA, DeBakey I as an intramural hematoma was established. The patient still suffered from refractory backpain. It was decided to perform emergency surgery with ascending aortic and complete aortic arch repair with frozen elephant trunk (FET) to treat both the ascending and proximal descending aorta.
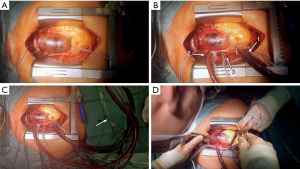
Emergency sternotomy was performed and the pericardium opened. Since the RSA could not be used for cannulation and central aortic cannulation is our first choice for arterial access in acute dissection cases, the ascending aorta and aortic arch were inspected carefully for direct access. As seen in the CTA, direct access to the true lumen was limited. Epiaortic sonography and transesophageal echocardiography confirmed a circular intramural hematoma in the ascending aorta and aortic arch. The decision was made to perform a direct “open” cannulation of the true lumen with temporary flush RCP to avoid unprotected cannulation of the true lumen through the thrombosed false lumen. Purse string sutures were placed on the right atrial appendage (RAA), the SVC and the right superior pulmonary vein (RSPV). The surgical field was flooded with CO2. The RAA was cannulated using a standard two-stage venous cannula (Medtronic, 36–46 French). The SVC was cannulated using a 90°-metal cannula (Medtronic, 24 French), which was connected via a Y-connector to the main arterial line. A vent catheter was placed in the RSPV (Figure 2). The aorta and pulmonary trunk were carefully divided to establish a position for later aortic clamping.
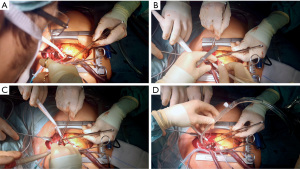
The heart was fibrillated and venous drainage as well as LA venting was started. After arterial pressure dropped to <20 mmHg, the ascending aorta was transected approximately 3 cm proximal to the innominate artery to allow for later clamping of the aorta. The SVC was occluded towards the RA and retrograde cerebral “flush” perfusion [flush RCP; approximately 500–1,000 mL/min; central venous target pressure (CVP) ≤40 mmHg; blood cooling started with target temperature of 20 ℃] was commenced (Figure 3). The true lumen of the aorta was cannulated over a wire in the proximal aortic arch under direct visual control through the opened aorta. The puncture site of the true lumen was inspected from inside the aorta to avoid embolization of thrombotic material from the intramural hematoma. After aortic cannulation, the arterial line was flushed to deair the line and fill the aortic arch via antegrade and retrograde perfusion (Figure 4). The thrombotic material was removed from the aortic wall at the clamping site and the aorta was clamped proximal to the aortic cannula after thoroughly deairing the aortic arch. Near infrared spectroscopy (NIRS) was used to measure regional cerebral oxygen saturation (rSPO2). NIRS values remained at baseline throughout the cannulation and RCP period (NIRS immediately before establishing antegrade flow: right rSPO2 =63%, left rSPO2 =62%). After establishing antegrade flow RCP was stopped and the RCP line clamped (Figure 5). Venous drainage from the upper body was reestablished by opening the SVC towards the RA. Arterial blood flow into the aortic arch was increased to the target output value and the patient was cooled for later total aortic arch repair. After direct open aortic cannulation with flush RCP the aortic root was opened and blood cardioplegia was delivered in an antegrade fashion via the coronary ostia.
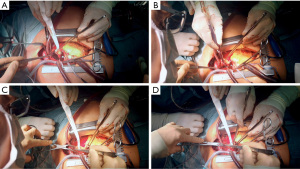
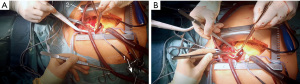
The video shows the process of direct open aortic cannulation with flush RCP in real time. Cannulation from opening of the aorta until clamping took 3:16 min including removal of thrombotic material from the false lumen.
The patient underwent aortic root reconstruction using the neomedia technique (PE patch from the PE prosthesis used for aortic replacement) followed by a supracommissural replacement of the ascending aorta (30 mm PE prosthesis, Terumo Aortic, Scotland). After reaching the target hypothermic circulatory arrest (HCA) temperature of 24 ℃, the ascending aortic graft was cannulated with a root cannula as described previously (12) and the vented heart was perfused with non-cardioplegic blood during the aortic arch procedure to reduce cardiac ischemia time (12). Total aortic arch repair was performed using a branched FET graft (Thoraflex Hybrid 30-32-100B, Terumo Aortic, Scotland) as described previously (13). The patient had an uneventful postoperative course without neurological sequelae and was discharged in good health on the eleventh postoperative day (November 25th, 2019). Discharge CTA showed a sufficient morphological result (Figure 6). Follow up is now 27 months. Recent CTA control shows a favourable result.
Discussion
Direct cannulation of the ascending aorta or aortic arch has been proposed by several groups to manage arterial access in AADA (2,6,7). Although central cannulation is our first priority in acute dissection cases, we refrain from cannulating the true lumen through the false lumen, especially if it is completely or partially thrombosed. Cannulation of the RSA may be an alternative in these cases. It is routinely used as the standard access route for arterial cannulation in aortic arch surgery in most centers (1,3). However, RSA cannulation may not be an option (e.g., in hemodynamically unstable patients or if the RSA is involved in the dissection, as in the case presented here). Some centers have suggested an open cannulation technique of the true lumen in these situations (8-10). Although central cannulation has not shown to result in more neurological complications than femoral cannulation (14), open cannulation poses a risk of embolization by air, debris or thrombotic material (2). We therefore have adapted the method of direct open cannulation of the true lumen: our technique adds temporary RCP to retrogradely “flush” the head vessels (flush RCP) and eliminate air, debris and thrombotic material from the supraaortic arteries during direct open cannulation of the aorta. In comparison to established techniques of RCP during aortic arch repair (11,15) that use RCP pressures of 25–30 mmHg, we suggest to use a large 90° SVC cannula as it is routinely used for bicaval cannulations. It allows fast increase in flow to ensure retrograde flushing. We keep RCP pressure (as measured over the central venous line) below 40 mmHg. In a study by Ganzel et al. RCP pressures of up to 40 mmHg were reported to be safe and resulted in an cerebral oxygen desaturation of only 0.4%/min (16). This is in line with our finding that NIRS sPO2 values typically remain at baseline during open aortic cannulation under flush RCP. RCP pressures of 40 mmHg mostly correspond to a flow of <1,000 mL/min, depending on whether the azygos vein is included into the RCP perfusion territory or not. For short periods, RCP flow may be increased to a maximum of 1,500 mL/min for flushing. However, perfusion pressures of >40 mmHg should be avoided to reduce the risk of cerebral edema (15).
We want to make clear that we do not advocate using RCP as an isolated method for cerebral protection, since SACP should be used for longer periods of circulatory arrest during complex aortic arch procedures (1,3). However, temporary RCP is an elegant adjunct to reduce the risk of emboli during open cannulation and aortic arch preparation before SACP is safely established (11).
In contrast to recently described methods of direct open cannulation of the aorta in acute dissections, we suggest cannulating the aortic arch over a wire and clamp the aorta proximally. To reduce the circulatory arrest time during open cannulation, other groups have suggested to place the aortic cannula directly through the transected aorta into the true lumen and snare the aorta with a tourniquet (8-10). However, with this technique the risk of leakages is high and the perfusion cannula typically obstructs the exposition of the aortic root. With the addition of flush RCP one can take the short period of time to place the arterial cannula over a wire into the aortic arch and clamp the ascending aorta. This helps to create an unobstructed surgical field and allows arterial access in a fast, predictable and reproducible fashion.
If one decides to use the open direct aortic cannulation technique in cases of intramural hematoma, as described here, thorough removal of thrombus from the clamping site is mandatory. This adds approximately another 60–80 seconds of circulatory arrest time. Without the need for thrombus removal, open direct aortic cannulation with RCP can be routinely achieved in less than 3 min (Video 1).
Time from opening the aorta until the start of antegrade cardioplegia was 5 min in our case. We have not observed myocardial damage under these conditions. Retrograde cardioplegia might be another option to protect the heart.
In our view, open direct aortic cannulation is an elegant method in selected patients, in which standard cannulation methods cannot be performed safely (8-10). However, open direct aortic cannulation should be combined with temporary RCP to minimize the risk of cerebral embolic events.
Limitations: Direct “open” aortic cannulation during normothermic circulatory arrest should remain limited to selected AADA cases that are not amenable for standard arterial cannulation techniques. Even if direct open aortic cannulation is combined with temporary RCP to flush air and debris from supraaortic arteries, the phase of normothermic circulatory arrest should be kept below 5 min to prevent organ damage. During this period the heart is also unprotected. One might consider retrograde cardioplegia administration to improve myocardial protection. Further studies are necessary to validate the benefits of this cannulation technique.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Roberto Di Bartolomeo, Davide Pacini and Mohamad Bashir) for the series “Best Video Presentation Prize for the 10th Postgraduate Course on ‘Surgery of the Thoracic Aorta’ in Bologna” published in Journal of Visualized Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jovs.amegroups.com/article/view/10.21037/jovs-21-4/rc
Peer Review File: Available at https://jovs.amegroups.com/article/view/10.21037/jovs-21-4/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.com/article/view/10.21037/jovs-21-4/coif). The series “Best Video Presentation Prize for the 10th Postgraduate Course on ‘Surgery of the Thoracic Aorta’ in Bologna” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in the case were in accordance with the standards of the institutional ethics committee and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- De Paulis R, Czerny M, Weltert L, et al. Current trends in cannulation and neuroprotection during surgery of the aortic arch in Europe. Eur J Cardiothorac Surg 2015;47:917-23. [Crossref] [PubMed]
- Abe T, Usui A. The cannulation strategy in surgery for acute type A dissection. Gen Thorac Cardiovasc Surg 2017;65:1-9. [Crossref] [PubMed]
- Erbel R, Aboyans V, Boileau C, et al. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2873-926. [Crossref] [PubMed]
- Ren Z, Wang Z, Hu R, et al. Which cannulation (axillary cannulation or femoral cannulation) is better for acute type A aortic dissection repair? A meta-analysis of nine clinical studies. Eur J Cardiothorac Surg 2015;47:408-15. [Crossref] [PubMed]
- Benedetto U, Mohamed H, Vitulli P, et al. Axillary versus femoral arterial cannulation in type A acute aortic dissection: evidence from a meta-analysis of comparative studies and adjusted risk estimates. Eur J Cardiothorac Surg 2015;48:953-9. [Crossref] [PubMed]
- Minatoya K, Karck M, Szpakowski E, et al. Ascending aortic cannulation for Stanford type A acute aortic dissection: another option. J Thorac Cardiovasc Surg 2003;125:952-3. [Crossref] [PubMed]
- Khaladj N, Shrestha M, Peterss S, et al. Ascending aortic cannulation in acute aortic dissection type A: the Hannover experience. Eur J Cardiothorac Surg 2008;34:792-6; disussion 796.
- Jakob H, Tsagakis K, Szabo A, et al. Rapid and safe direct cannulation of the true lumen of the ascending aorta in acute type A aortic dissection. J Thorac Cardiovasc Surg 2007;134:244-5. [Crossref] [PubMed]
- Conzelmann LO, Weigang E, Mehlhorn U, et al. How to do it: direct true lumen cannulation technique of the ascending aorta in acute aortic dissection type A. Interact Cardiovasc Thorac Surg 2012;14:869-70. [Crossref] [PubMed]
- Kitamura T, Torii S, Kobayashi K, et al. Samurai cannulation (direct true-lumen cannulation) for acute Stanford Type A aortic dissection. Eur J Cardiothorac Surg 2018;54:498-503. [Crossref] [PubMed]
- Pochettino A, Brinkman WT, Moeller P, et al. Antegrade thoracic stent grafting during repair of acute DeBakey I dissection prevents development of thoracoabdominal aortic aneurysms. Ann Thorac Surg 2009;88:482-9; discussion 489-90. [Crossref] [PubMed]
- Martens A, Koigeldiyev N, Beckmann E, et al. Do not leave the heart arrested. Non-cardioplegic continuous myocardial perfusion during complex aortic arch repair improves cardiac outcome. Eur J Cardiothorac Surg 2016;49:141-8. [Crossref] [PubMed]
- Shrestha M, Kaufeld T, Beckmann E, et al. Total aortic arch replacement with a novel 4-branched frozen elephant trunk prosthesis: Single-center results of the first 100 patients. J Thorac Cardiovasc Surg 2016;152:148-159.e1. [Crossref] [PubMed]
- Ma H, Xiao Z, Shi J, et al. Aortic arch cannulation with the guidance of transesophageal echocardiography for Stanford type A aortic dissection. J Cardiothorac Surg 2018;13:106. [Crossref] [PubMed]
- Usui A, Abe T, Murase M. Early clinical results of retrograde cerebral perfusion for aortic arch operations in Japan. Ann Thorac Surg 1996;62:94-103; discussion 103-4. [Crossref] [PubMed]
- Ganzel BL, Edmonds HL Jr, Pank JR, et al. Neurophysiologic monitoring to assure delivery of retrograde cerebral perfusion. J Thorac Cardiovasc Surg 1997;113:748-55; discussion 755-7. [Crossref] [PubMed]
Cite this article as: Martens A, Beckmann E, Kaufeld T, Fleissner F, Arar M, Natanov R, Korte W, Shrestha ML. Direct open aortic cannulation under temporary retrograde cerebral flush perfusion in acute aortic dissection Stanford type A—a case report. J Vis Surg 2023;9:24.


