
Role of tumor reduction surgery in multimodality therapy for advanced thymoma—case series
Introduction
In patients with a thymoma, complete surgical resection is the main determinant of outcome and postoperative survival. However, for those in an advanced stage, complete resection is not possible without causing damage to a main organ (1), thus no optimal treatment method has been established for thymoma patients with tumor invasion to vital organs like the aorta and heart. Surgical resection that includes a mediastinal great vessel structure, such as the aorta or main pulmonary artery (PA) trunk, in cases of thymic malignancy is challenging, because extracorporeal circulation is generally required (2,3). Furthermore, these patients often have pleural dissemination, thus a pathological complete resection for an advanced thymoma is not possible for those cases.
When considering the potential for a more favorable outcome for treatment of a thymoma as compared to thymic cancer patients, surgical debulking of a disseminated tumor as well as the primary tumor is thought to be acceptable for an invasive thymoma (4). Debulking surgery minimizes tumor size and the area for postoperative radiotherapy (PORT), resulting in less damage to adjacent tissues during PORT, thus it may be applicable for patients with advanced-stage thymoma in whom extensive radiotherapy (RT) will be required (5).
Although debulking surgery has been defined as a maximally tolerable resection or at least 90% resection of the main lesion (6), we have rarely experienced cases in which a less than 90% resection of the tumor is performed, termed “tumor reduction surgery”. For the present study, tumor reduction surgery was defined as removal of as much of the tumor as possible in cases of unresectable advanced thymoma. To elucidate the effectiveness of reduction surgery for unresectable thymoma, we retrospectively analyzed our previous cases. We present the following article in accordance with the STROBE reporting checklist (available at https://jovs.amegroups.com/article/view/10.21037/jovs-21-59/rc).
Methods
Patients
Between 2005 and 2018, 51 patients with a Masaoka stage III or IV thymoma underwent surgical resection at Osaka University Hospital. Of those, intended tumor reduction surgery in which a less than 90% resection of the primary tumor was performed followed by RT was performed in six and those cases were retrospectively reviewed. Disease stage was determined according to the Masaoka staging system for thymic epithelial tumors and tumor-node-metastasis (TNM) classification (7,8). Pathological diagnosis was determined according to the World Health Organization (WHO) classification (9). This study was conducted in accordance with the Declaration of Helsinki (revised in 2013) and approved by the Institutional Review Board of Osaka University (Approval No. 18518). Need for individual consent was waived, as this was a retrospective analysis and data were accessed after masking patients’ identity.
Treatment
All patients were preoperatively diagnosed with an invasive thymoma with or without pleural dissemination, and their treatment strategy was discussed by our multidisciplinary team. At our hospital, the following criteria are used for indicating preoperative chemotherapy: (I) detection of great vessel and/or adjacent organ invasion, or encirclement based on radiological examination findings and/or detection of a large amount of dissemination in preoperative radiological examinations; (II) Eastern Cooperative Oncology Group (ECOG) performance status 0 or 1; and (III) in patients with autoimmune disease, stable disease (SD) and chemotherapy considered to be tolerable (10). When invasion of adjacent great vessels or the heart was obvious, and dissemination lesions were considered to be controllable by a pleurectomy procedure after chemotherapy, tumor reduction surgery including resection of the primary tumor as well as dissemination lesions as much as possible was planned. Otherwise, additional chemotherapy was considered for disease control. For avoiding injury to vital organs such as the aortic arch and PA trunk during surgery, the intention was to leave the tumor adjacent to them. The surgery for patients with advanced thymoma was performed as a median sternotomy or that with a lateral thoracotomy (11,12).
A representative tumor reduction surgery procedure in one of the patients is presented in Video 1. A 50-year-old male underwent surgery for stage IVa thymoma following systemic chemotherapy. Preoperative computed tomography (CT) findings revealed that the tumor was located in the mediastinum surrounding the aortic arch as well as hilum of the left lung, thus it was resected along with the pericardium and left upper lobe (Figure 1). A median sternotomy and lateral thoracotomy were performed. Although the tumor was resected along with the pericardium and left upper lobe, a portion remained on the wall of the aortic arch. Because of invasion of the aortic arch, as well as root of the left common carotid artery and subclavian artery, the residual tumor was removed as much as possible. Clips were placed at the site of the residual tumor at the end of the reduction surgery procedure (Video 1). In another case, tumor reduction surgery was performed under a cardiopulmonary bypass for acquisition of a surgical view in the pericardium. The patient was a 38-year-old male who showed extensive tumor development in the pericardium after undergoing systemic chemotherapy, which indicated the presence of cardiac compression and risk of mortality (Figure 2). A median sternotomy and lateral thoracotomy were performed. After opening the pericardium, massive bloody pericardial effusion was aspirated, the tumor was detached from the superior vena cava (SVC), and the left brachiocephalic vein was dissected. The upper lobe of the left lung had been invaded, thus a partial resection was performed, with the tumor detached along the ascending aorta and divided halfway through. Continuity of tumor invasion from the PA trunk to aorta dorsal side was noted, and surgery was continued under a cardiopulmonary bypass to maintain the visual field and for patient safety. The remaining tumor was then resected in pieces along the PA trunk. Invasion of the left coronary artery and left atrium was noted, with no resection performed there (Video 2).
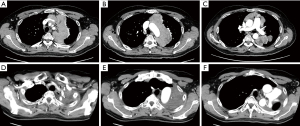
In each of the present cases, reduction surgical surgery was performed without aortic replacement, PA trunk replacement, or heart resection, thus the residual tumor remained adjacent to vital organs following surgery. At the end of the procedure, clips were placed at the site of the residual tumor to facilitate identification of the target volume for PORT given for the residual tumor within 3 months.
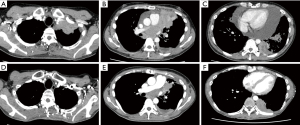
Tumor reduction surgery minimizes the area of PORT, resulting in less damage to adjacent tissues during that therapy. However, when that surgical procedure seems to be intolerable for a patient due to poor cardiac or respiratory function, or systemic disease, we select conservative treatment such as chemotherapy and/or RT for advanced thymoma patients. Representative cases are presented in Figure S1. For one of those with advanced thymoma, reduction surgery was planned. A thoracotomy was performed, during which innumerable small intrapericardial lesions and pleural dissemination, as well as the primary tumor were found invading the PA trunk, thus an exploratory thoracotomy was selected because tumor reduction surgery did not affect the range of PORT due to uncontrollable disseminations. Another patient had an advanced thymoma invading the heart after systemic chemotherapy. In this case, the tumor was mainly located in intracardiac space, a life-threatening condition. Respiratory function was too poor for the patient to undergo surgery under a cardiopulmonary bypass and we considered that tumor reduction outside the heart might not have a beneficial effect, thus chemotherapy and immunotherapy were performed.
Follow-up
Follow-up assessments were conducted every 3 to 6 months after treatment for the first year, and every 6 months thereafter, with physical examination and chest CT results used. SD was defined as disease controlled and no evidence of enlargement of the residual tumor. Survival time was calculated from the date of surgery to date of death or final follow-up examination.
Tumor reduction rate
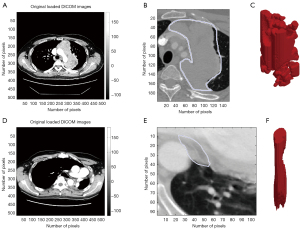
Tumor resection rate was calculated based on CT findings, and resection-targeted tumor volume change was compared between pre- (after induction chemotherapy) and post-surgery measurements. Volumetric measurements were semi-automatically obtained using a modified commercially available software package (LISIT, Co., Ltd., Tokyo, Japan) by one of the authors (YM) who was not informed regarding the clinical characteristics of the patients, as described previously in another study presented by our institution (13). Briefly, after a rough tracing of the tumor shown in a single CT slice with the computer cursor, tumor volume was calculated automatically (Figure 3). Tumor reduction rate was then determined using the following formula: (preoperative tumor volume − tumor volume after surgery)/preoperative tumor volume × 100. In patients with stage IVa thymoma, the disseminated lesions were resected as much as possible with a partial pleurectomy procedure.
Results
Patient characteristics are presented in Table 1. The study population included four males and two females, with a mean age of 49 (range, 38–68) years. Preoperative Masaoka stage was III in one and IVa in five, while histological type was B2 and B3 in four and two, respectively. Preoperative chemotherapy was performed in all cases except Case 5, because of encephalitis of unknown origin. Using the calculation formula described above in the Methods section, the tumor reduction rates, based on comparisons of just before and after surgery, ranged from 29% to 89%. Two patients underwent a gross tumor volume removal of more than 50% and four of less than 50%, while pleural or pericardial disseminations were macroscopically resected using a pleurectomy in all except for Case 6. The grossly reduced tumor was located in the aorta arch in four, arch vessels in one, and PA trunk in one. Postoperatively, two developed respiratory complications. Serial CT findings for all patients obtained in pre- and post-operative examinations, as well as a few years after the operation are shown in Figure S2. Surgery was performed under a cardiopulmonary bypass only for Case 6, with pleural disseminations in the dorsal thoracic cavity left to avoid excessive surgical stress (Video 2, Table 1, Figure S2).
Table 1
| Case | Age | Gender | Preope Masaoka stage | Histology (WHO type) | Preope CTx | Courses of CTx | Approach | Ope time (min) | Blood loss (g) | Transfusion (mL) | Residual tumor | Postope complication | Tumor reduction rate | Masaoka stage | TNM stage | Postope RT (Gy) | Postope CTx | Disease control | Tumor reduction rate* | Survival (months) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 40 | F | IVa | B2 | ADOC | 2 | Sternotomy | 315 | 880 | 0 | Ao arch | None | 35.1 | IVa | T4N0M1a (IVa) | 50 | TJ | PD** | 76.9 | 81 |
| 2 | 42 | F | III | B2 | ADOC | 4 | Neck + sternotomy | 450 | 1,320 | 1,120 | Arch vessels | None | 35.5 | III | T4N0M0 (IIIB) | 50 | – | SD | 87.2 | 147 |
| 3 | 68 | M | IVa | B3 | ADOC | 5 | Sternotomy | 215 | 275 | 0 | PA trunk | None | 40.9 | IVa | T4N0M1a (IVa) | 60 | – | SD | 78.9 | 149 |
| 4 | 50 | M | IVa | B2 | CDDP + VP16 | 4 | Sternotmy + thoracotomy | 567 | 2,280 | 1,800 | Ao arch | None | 89.1 | IVa | T4N0M1a (IVa) | 60 | – | SD | 98.4 | 40 |
| 5 | 53 | M | IVa | B2 | – | 0 | Sternotmy + thoracotomy | 613 | 2,870 | 2,180 | Ao arch | Atelectasis | 29.1 | IVa | T4N0M1a (IVa) | 60 | – | SD | 69.1 | 27 |
| 6 | 38 | M | IVa | B3 | ADOC | 4 | Sternotmy + thoracotomy | 368 | 2,730 | 1,400 | Ao arch | Respitatory failure | 52.4 | IVa | T4N0M1a (IVa) | 60 | – | DOO | 71.3 | 11 |
*, tumor reduction rate was calculated using the latest CT findings; **, the disease was treated with chemotherapy and controlled. Prepe, preoperative, WHO, World Health Organization; CTx, chemotherapy; ADOC, adriamycin + cisplatin + vincristine + cyclophosphamide; CDDP, cisplatin; VP-16, etoposide; ope, operation; RT, radiotherapy; postope, postoperative; Ao, aortic; PA, pulmonary artery; TJ, paclitaxel + carboplatin; TNM, tumor-node-metastasis; PD, progressive disease; SD, stable disease; DOO, died of other disease.
Postoperative Masaoka stage was III in one and IV in five patients, while 8th edition TNM stage was IIIB in one and IVa in five patients. All underwent PORT for mediastinal lesions, with a dose to the target of 50 Gy in two and 60 Gy in four. Consolidation chemotherapy with four courses of carboplatin and paclitaxel was given to a single patient after resection of massive pleural disseminations. This patient had re-growth lesions in the pleural cavity, thus received chemotherapy with docetaxel for controlling the disease. None of the other patients received adjuvant therapy other than PORT. One died at 11 months after the operation of respiratory failure due to atypical mycobacterial disease after RT, while the other patients were alive with controlled disease at the time of writing for a mean period of 89 (range, 27–149) months. The most recent CT findings showed tumor reduction rates ranging from 69% to 98%, based on the calculation formula described in the Methods section (Figure S2).
Discussion
Here, results of tumor reduction surgery for patients with unresectable thymoma are presented. When treating a case of unresectable thymoma, it is very important to control the disease by use of multimodality treatment. Based on our experience with the present patients, we propose tumor reduction surgery as a part of multimodality treatment for patients with an unresectable thymoma. Five of the six patients who underwent tumor reduction surgery were alive at the time of writing with a controlled tumor and sufficient tumor reduction rates noted.
While most studies have not found any survival benefit of debulking surgery for patients with advanced thymoma, Hamaji et al. reported a meta-analysis of published retrospective cohort studies indicating that debulking surgery for unresectable thymoma may be associated with improved overall survival and recommended that it should be considered for affected patients (4). Another report noted that for cases of unresectable or incomplete resected thymoma, not debulking surgery but definitive RT with a dose greater than 54 Gy may lead to long-term tumor control, though there is still no evidence supporting debulking surgery for treatment of unresectable thymoma (6). Each of the present patients had an extremely advanced thymoma showing extensive invasion to the aorta, PA trunk, and heart, thus complete resection was impossible based on preoperative workup results. The ability to perform lesion resection is dependent on the positional relationship between the primary tumor and vital organs. However, it is difficult for the attending surgeon to remove most of a tumor that has widely invaded a great vessel or the heart while also avoiding injury to such vital organs. Similar to debulking surgery for thymoma, even if the tumor reduction rate is less than 90% of the whole tumor, the aim of tumor reduction surgery is to minimize tumor size as well as remove dissemination, thus reducing the required amount of RT dosage to lower the amount of damage caused by that to adjacent tissues is necessary. Although a previous study found that survival of patients with advanced thymoma did not seem to be affected by surgery and also noted that the role of surgery as compared with definitive RT remained unclear (14), we consider that reduction surgery is a valuable option for advanced thymoma with disseminations. In such cases, irradiation of the residual mediastinal tumor after resection of disseminations is normally planned. The amount of RT dosage as well as irradiation region can be predicted before treatment, which should be taken into account to decide a treatment strategy. Indeed, three of the present patients with stage IVa thymoma showed SD without re-growth of disseminations after undergoing resection. In general, adjuvant RT or chemoradiotherapy should be considered for Masaoka stage III or above, though there is no evidence that adjuvant chemotherapy improves survival in completely resected stage III and IV thymoma cases (15). In the present series, adjuvant chemotherapy was given to a single patient following a pleurectomy for massive pleural disseminations, after which re-growth of pleural disseminations was noted and then continuous systemic chemotherapy was given (Case 1). None of the other patients received adjuvant therapy other than PORT, as no evidence of a beneficial effect of adjuvant chemotherapy on survival has been presented.
For patients at our hospital with stage III thymoma who did not agree to undergo surgery, definitive RT is performed when poor performance status is present. We were not able to clarify the clinical outcomes of concurrent sequential chemoradiotherapy or definitive RT for patients with an unresectable thymoma, because they were often treated at other hospitals. However, a previous report of six thymoma patients (one stage 1 or 2, one stage III, three stage IVa, one stage IVb) noted median and progression-free survival after chemoradiotherapy of 64.1 and 38.2 months, respectively (16). It will be necessary to perform a prospective controlled trial of patients with an unresectable thymoma who undergo reduction surgery as part of multimodality treatment to definitively elucidate the results of chemoradiotherapy.
The risks of surgery in patients who receive multimodality treatment must be carefully assessed prior to initiation of treatment. One of the present patients died of respiratory failure within 1 year after treatment. In that case, extensive tumor development was seen in the pericardium, indicating that cardiac compression and mortality were possible, thus we performed tumor reduction surgery in consideration of the young age of the patent as a lifesaving procedure.
Limitations
This study has some limitations, including a sample size too small to fully discuss factors related to prognosis. Additionally, the enrolled patients underwent planned tumor reduction surgery and their clinical records were retrospectively investigated, thus case-selection bias was inevitable. Finally, we were not able to compare the outcomes of patients with tumor reduction surgery followed by RT with those of patients treated with definitive RT without surgery.
Conclusions
For patients with advanced thymoma, multimodality treatment, including chemotherapy, RT, and surgery, is often applied as curative therapy. Tumor reduction surgery as part of multimodality therapy for advanced thymoma may be effective in select cases.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jovs.amegroups.com/article/view/10.21037/jovs-21-59/rc
Data Sharing Statement: Available at https://jovs.amegroups.com/article/view/10.21037/jovs-21-59/dss
Peer Review File: Available at https://jovs.amegroups.com/article/view/10.21037/jovs-21-59/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.com/article/view/10.21037/jovs-21-59/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (revised in 2013) and approved by the Institutional Review Board of Osaka University (Approval No. 18518). Need for individual consent was waived, as this was a retrospective analysis and data were accessed after masking patients’ identity.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg 2003;76:878-84; discussion 884-5. [Crossref] [PubMed]
- Ried M, Neu R, Schalke B, et al. Radical surgical resection of advanced thymoma and thymic carcinoma infiltrating the heart or great vessels with cardiopulmonary bypass support. J Cardiothorac Surg 2015;10:137. [Crossref] [PubMed]
- Wright CD. Extended resections for thymic malignancies. J Thorac Oncol 2010;5:S344-7. [Crossref] [PubMed]
- Hamaji M, Kojima F, Omasa M, et al. A meta-analysis of debulking surgery versus surgical biopsy for unresectable thymoma. Eur J Cardiothorac Surg 2015;47:602-7. [Crossref] [PubMed]
- Attaran S, Acharya M, Anderson JR, et al. Does surgical debulking for advanced stages of thymoma improve survival? Interact Cardiovasc Thorac Surg 2012;15:494-7. [Crossref] [PubMed]
- Fan C, Ge H, Zhang S, et al. Impact of Definitive Radiotherapy and Surgical Debulking on Treatment Outcome and Prognosis for Locally Advanced Masaoka-Koga stage III Thymoma. Sci Rep 2020;10:1735. [Crossref] [PubMed]
- Masaoka A, Monden Y, Nakahara K, et al. Follow-up study of thymomas with special reference to their clinical stages. Cancer 1981;48:2485-92. [Crossref] [PubMed]
- Detterbeck FC, Stratton K, Giroux D, et al. The IASLC/ITMIG Thymic Epithelial Tumors Staging Project: proposal for an evidence-based stage classification system for the forthcoming (8th) edition of the TNM classification of malignant tumors. J Thorac Oncol 2014;9:S65-72. [Crossref] [PubMed]
- Marx A, Chan JK, Coindre JM, et al. The 2015 World Health Organization Classification of Tumors of the Thymus: Continuity and Changes. J Thorac Oncol 2015;10:1383-95. [Crossref] [PubMed]
- Kanzaki R, Kanou T, Ose N, et al. Long-term outcomes of advanced thymoma in patients undergoing preoperative chemotherapy or chemoradiotherapy followed by surgery: a 20-year experience. Interact Cardiovasc Thorac Surg 2019;28:360-7. [Crossref] [PubMed]
- Fujiwara A, Funaki S, Ose N, et al. Surgical resection for advanced thymic malignancy with pulmonary hilar invasion using hemi-clamshell approach. J Thorac Dis 2018;10:6475-81. [Crossref] [PubMed]
- Shintani Y, Funaki S, Ose N, et al. Surgical management of thymic epithelial tumors. Surg Today 2021;51:331-9. [Crossref] [PubMed]
- Sato Y, Yanagawa M, Hata A, et al. Volumetric analysis of the thymic epithelial tumors: correlation of tumor volume with the WHO classification and Masaoka staging. J Thorac Dis 2018;10:5822-32. [Crossref] [PubMed]
- Kunitoh H, Tamura T, Shibata T, et al. A phase II trial of dose-dense chemotherapy, followed by surgical resection and/or thoracic radiotherapy, in locally advanced thymoma: report of a Japan Clinical Oncology Group trial (JCOG 9606). Br J Cancer 2010;103:6-11. [Crossref] [PubMed]
- Attaran S, McCormack D, Pilling J, et al. Which stages of thymoma benefit from adjuvant chemotherapy post-thymectomy? Interact Cardiovasc Thorac Surg 2012;15:273-5. [Crossref] [PubMed]
- Kashima J, Okuma Y, Murata H, et al. Chemoradiotherapy for unresectable cases of thymic epithelial tumors: a retrospective study. J Thorac Dis 2017;9:3911-8. [Crossref] [PubMed]
Cite this article as: Shintani Y, Funaki S, Miyashita Y, Ose N, Kanou T, Fukui E, Minami M. Role of tumor reduction surgery in multimodality therapy for advanced thymoma—case series. J Vis Surg 2022;8:33.


