
Tracheobronchoplasty for tracheobronchomalacia
Introduction
The goal of tracheobronchoplasty (TBP) in tracheobronchomalacia (TBM) patients is to restore a normal configuration of the airway by stabilizing the cartilaginous rings and plicating the redundant posterior membranous wall. Airway splinting was first introduced to treat cases of tracheal stenosis, especially in the pediatric population. These early interventions included procedures involving excision of thickened tissue with grafting and splint for six weeks as well as splitting of the larynx with a Teflon stent (1,2). Problems with granulation tissue formation and vocal cord damage were noted with each technique. There were also later attempts to use polyethylene mesh prosthesis for membranous wall tracheoplasty in patients with emphysema, but 4 out of 12 died from erosion of the prosthetic into surrounding structures (3).
One of the early case-series of TBP for TBM was from the Massachusetts General Hospital group, which reported on 14 patients who underwent right thoracotomy for posterior tracheobronchial splinting with polypropylene mesh (4). The authors demonstrated significant clinical improvement and an increase in mean predicted FEV1 from 51% to 73% (P=0.009). Using the same approach and a comparable technique with polypropylene mesh, Gangadharan and colleagues from Beth Israel Deaconess Hospital in Boston published their first case series in 2011 of 63 patients who underwent TBP. While there was no statistical improvement in pulmonary functions tests, patients reported significant improvement in functional status and respiratory questionnaires scores (5). The group later reported their experience with 161 patients who underwent TBP from 2002 until 2016 (6). Severe complications (Clavien-Dindo grade ≥IIIa) occurred in 38 patients (24%), including 27 (17%) who had respiratory failure. Median intensive care unit length of stay was 4 days and median total length of stay was 8 days. In-hospital mortality occurred in 2 patients, and 68% of patients were discharged home.
While these series of TBP were all performed through a right thoracotomy, one of the early reports of a minimally invasive TBP was published by Tse et al. (7). The authors reported 2 cases of video-assisted thoracoscopic surgical TBP combined with airway stent placement for the treatment of TBM patients. However, it was the advent of the robotic technology with its more precise wristed motion that opened new horizons for tracheobronchial surgery. Lazzaro and colleagues reported the first series of robotic assisted TBP in 42 patients out of 435 who underwent evaluation for TBM. The median operative time (249 min) and median total length of stay (3 days) were substantially lower when compared to historical open TBP series such as the one by Gangadharan and colleagues (the median length of stay of 8 days, of which 3 days were in intensive care) (5,8). Eight patient developed major complications, but there was no mortality at 90 days. Furthermore, patients did exhibit statistically significant improvement in FEV1 by 13.5% and peak expiratory flow rate by 21%. Of note, a significant proportion of patients in this report had underlying asthma (88%). This remains the largest robotic TBP series in the literature to date.
In this manuscript, we review technical considerations in the surgical treatment of TBM, and highlight our own experience with the open and robotic approaches. While the pathophysiology, clinical presentation and work-up of TBM is beyond the scope of this manuscript, it is important to stress that surgery should be reserved for severely symptomatic patients.
TBP via open thoracotomy
The patient undergoes general anesthesia and intubation with a single lumen endotracheal tube (ETT), which allows inspection of the airway, suctioning of secretions and collection of appropriate cultures if necessary. An epidural can be placed prior to induction and after discussion with the anesthesia team; alternatively, an intercostal nerve block can be performed in the operating room. A bronchial blocker is then placed in the right main stem bronchus. The patient is then placed in the left lateral decubitus position with the pressure points padded as these cases can be lengthy in patients who cannot tolerate single lung ventilation for prolonged periods. Short apnea episodes with deflation of the bronchial blocker will also be necessary to place the sutures in the right mainstem bronchus.
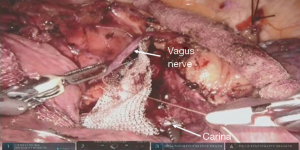
A generous right posterolateral thoracotomy is then performed and the chest cavity entered through the fourth intercostal space. We typically transect the fourth rib posteriorly to obtain adequate exposure, without resecting it. The right lung is retracted anteriorly and the mediastinal pleura dissected to expose the airway from the thoracic inlet to the first 4 cm of the mainstem bronchi on the left and the bronchus intermedius on the right. The azygos vein is divided and tied in the process, and the vagus nerve dissected and preserved throughout the procedure. The airway is dissected with care, and the lateral dissection is kept to a minimum as not to impair the blood supply. We then typically perform transverse measurements of the airway at the following levels: proximal trachea, distal trachea, right mainstem bronchus, bronchus intermedius, and left mainstem bronchus. Depending on the pathophysiology and whether or not we are dealing with expiratory dynamic airway collapse versus malacia of the cartilage with further widening of the coronal diameter during expiration, the airway can be downsized anywhere from 10% to 30%. A polypropylene non absorbable mesh is then cut accordingly, making sure to leave at least 3–4 mm on each side to avoid the sutures pulling through (Figure 1).
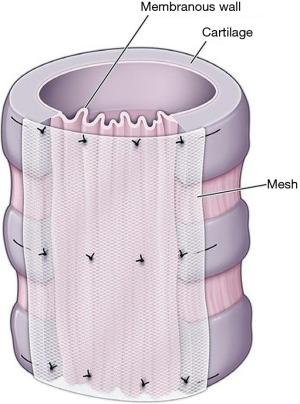
Using 4-0 polypropylene sutures on an RB1 needle, we place the distal tracheal sutures followed by the right mainstem bronchus sutures followed by a central membranous airway carinal suture and finally the left mainstem bronchus sutures. These sutures are placed in rows of four, and each is a surgical mattress going through the mesh, then through the airway, and then back through the mesh again. We space the suture a third of the way on the membranous airway, but closer to the lateral edges on the mesh side to have a more significant plication effect. After placing the first 13 sutures, we parachute the mesh onto the airway. The sutures are tied beginning with the lateral cartilaginous-membranous ones and then the 2 posterior membranous sutures in that sequence, as to not to cause any avulsion of the airway.
From thereafter, we continue placing the rows of sutures on the trachea going from caudad to cephalad towards the thoracic inlet. With each suture, care should be taken to avoid entering the airway lumen as much as possible. We advance in rows of 4 sutures every 5–7 mm on the airway side, and about 4 mm on the mesh side to ensure longitudinal plication effect as well (Figure 2).
We then place the sutures on the right mainstem bronchus, bronchus intermedius and left mainstem bronchus level in rows of 4 then of 3, as the transverse diameter of the airway becomes smaller. We make sure not to impinge the opening of the right upper lobe bronchus in the process. Of note that placement of the sutures can be guided throughout the procedure by intraoperative bronchoscopy through the single-lumen ETT. Intraluminal sutures can be removed and retaken appropriately.
At the end of the procedure, irrigation is undertaken, a thoracostomy tube placed in the posterior gutter and the thoracotomy is closed in the usual fashion. After the patient is placed back supine, flexible bronchoscopy is performed to assess the TBP and to suction any remaining secretions. Our aim is usually to extubate the patient in the OR.
Robotic-assisted TBP
The patient’s OR set up is similar to an open tracheoplasty, except that an epidural is usually unnecessary. We typically use the smallest size left sided double lumen ETT possible (35 or 37 Fr), although a bronchial blocker could also achieve appropriate isolation of the right lung. The first 8 mm port (camera) is usually placed slightly below and anterior to the scapular tip in the 8th intercostal space. After establishing a capnothorax to a pressure of 8 mmHg, we place the additional port as follows: an 8 mm robotic port 1 handbreadth from the initial one in the 6th intercostal between the mid and posterior axillary line (Maryland bipolar forceps/needle driver), an 8 mm port in the anterior axillary line in the 4th or 5th space (double fenestrated forceps), and a more posterior 8 mm port one hand breath from the initial port in the ninth intercostal space (fenestrated bipolar). We finally place a 12 mm assistant port right above the diaphragm in the posterior axillary line in the 8th or 9th space.
The principles of dissection and exposure of the trachea are very similar to the open technique.
Advantages of the robotic approach over the open one include the ability to better expose the left main stem bronchus, an excellent visualization throughout the procedure, and significant ergonomic benefits to the operating surgeon.
The splinting is achieved by placing 3 separate pieces of a polypropylene mesh for the trachea, right main stem and left main stem bronchi, and these are them together at the end at a level above the carina. We use absorbable polyglactin 4-0 sutures on an RB1 needle throughout the procedure, partially because they are easier to handle robotically when compared with polypropylene sutures. All the sutures are placed in a vertical mattress fashion going through the polypropylene mesh then through the airway and back through the mesh again, similar to the open approach (Figure 3). The membranous wall is plicated and the airway downsized appropriately throughout the splinting process. Of note that the bronchial balloon has to be deflated and the ETT pulled back when placing the left main stem bronchial sutures, and the tracheal balloon deflated when placing the more proximal tracheal sutures. Similar to the open approach, periods of apnea can be necessary when placing the sutures and even tying the knots. An illustration of our robotic technique is presented in Video 1.
Temple University Hospital’s TBP experience
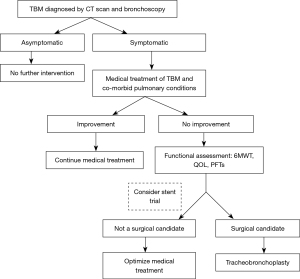
Thirty-four patients were referred to our TBM program. They were evaluated in a multidisciplinary fashion involving pulmonary medicine, thoracic surgery, gastroenterology, ear, nose, and throat (ENT) and speech therapy. Our management algorithm is shown in Figure 4. Patients with symptomatic TBM are first optimized from a medical and pulmonary standpoint. This usually includes control of any reflux symptoms, nebulizer treatment, mucolytics and antibiotics when appropriate. If patients remain symptomatic despite medical treatment, they are then evaluated for surgical TBP. We currently reserve stent placement to equivocal cases before deciding who might benefit from surgery. We believe that stents are associated with significant complications and the interpretation of symptoms after placement is still prone to subjectivity. The role of stenting in TBM is discussed by Majid and colleagues in this series.
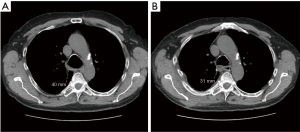
We performed a total of 8 TBPs, 5 through a right thoracotomy approach and the last 3 done were done robotically. Five patients were female, mean age 58 (range, 32–73) years, mean BMI 32.8 (range, 25–38). One patient had tracheobronchomegaly or Mounier-Kuhn syndrome with severe recurrent bronchitis, 2 patients had chronic obstructive pulmonary disease (COPD), 2 had asthma, 4 had sleep apnea and 4 had gastro-esophageal reflux disease (GERD). The mean operative time was 653 min for the open approach and 494 min for the robotic one. No patient required re-intubation or ventilator support of more than 2 days. The mean length of stay was 7.2 days for the thoracotomy group and 6.7 days for the robotic one. FEV1 improved from 2.02±0.53 pre-operatively (mean ± SD) to 2.41±0.65 L post-operatively; DLCO improved from 62%±13.4% to 72%±27%. Examples of post-operative bronchoscopy images and chest CT-scan cuts demonstrating significant improvement in the dynamic airway collapse are shown in Figures 5-7.
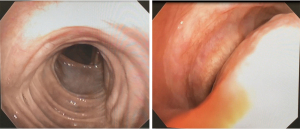
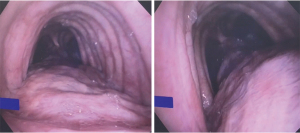
Conclusions
TBP should be reserved for severely symptomatic patients with TBM. Regardless of approach, the main goal of posterior membranous wall splinting is to reconfigure the airway to the closest possible normal shape and prevent excessive collapse. The robotic approach offers significant advantages over a thoracotomy, and seems to be associated with a shorter hospital stay and overall less morbidity. Long-term outcomes should be closely monitored to ensure the durability of the splinting in a patient population which is prone to respiratory infections and exacerbation of the underlying pulmonary condition. This is particularly true in the robotic approach, where use of absorbable sutures seems to be preferred over non-absorbable sutures. Performing the surgery at expert centers that offer a multidisciplinary evaluation is paramount to achieve excellent results. A national registry or database for TBM patients would also be complementary to ensure appropriate longitudinal follow-up.
Acknowledgments
Funding: The study was supported by the Fox Chase Cancer Center Core Grant (NIH P30 CA006927).
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Visualized Surgery for the series “Tracheobronchoplasty”. The article has undergone external peer review.
Peer Review File: Available at https://jovs.amegroups.com/article/view/10.21037/jovs-21-56/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.com/article/view/10.21037/jovs-21-56/coif). The series “Tracheobronchoplasty” was commissioned by the editorial office without any funding or sponsorship. CTB served as the unpaid Guest Editor of the series. AEA served as the unpaid Guest Editor of the series and serves as the unpaid editorial board member of Journal of Visualized Surgery from July 2020 to June 2022. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the Helsinki Declaration (as revised in 2013). The manuscript is waived from patient informed consent according to the ethics committee or institutional review board.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Montgomery WW. The surgical management of supraglottic and subglottic stenosis. Ann Otol Rhinol Laryngol 1968;77:534-46. [Crossref] [PubMed]
- Aboulker P. Treatment of tracheal stenosis. Probl Actuels Otorhinolaryngol 1968;275-95. [PubMed]
- Rainer WG, Newby JP, Kelble DL. Long term results of tracheal support surgery for emphysema. Dis Chest 1968;53:765-72. [Crossref] [PubMed]
- Wright CD, Grillo HC, Hammoud ZT, et al. Tracheoplasty for expiratory collapse of central airways. Ann Thorac Surg 2005;80:259-66. [Crossref] [PubMed]
- Gangadharan SP, Bakhos CT, Majid A, et al. Technical aspects and outcomes of tracheobronchoplasty for severe tracheobronchomalacia. Ann Thorac Surg 2011;91:1574-80; discussion 1580-1. [Crossref] [PubMed]
- Buitrago DH, Majid A, Alape DE, et al. Single-Center Experience of Tracheobronchoplasty for Tracheobronchomalacia: Perioperative Outcomes. Ann Thorac Surg 2018;106:909-15. [Crossref] [PubMed]
- Tse DG, Han SM, Charuworn B, et al. Video-assisted thoracoscopic surgical tracheobronchoplasty for tracheobronchomalacia. J Thorac Cardiovasc Surg 2011;142:714-6. [Crossref] [PubMed]
- Lazzaro R, Patton B, Lee P, et al. First series of minimally invasive, robot-assisted tracheobronchoplasty with mesh for severe tracheobronchomalacia. J Thorac Cardiovasc Surg 2019;157:791-800. [Crossref] [PubMed]
Cite this article as: Bakhos CT, Magarinos J, Bent D, Petrov R, Abbas AE. Tracheobronchoplasty for tracheobronchomalacia. J Vis Surg 2022;8:15.



