Thoracoscopic subxiphoid right S9+10 segmentectomy: a case report
Introduction
Thoracoscopic segmentectomy is a quite appropriate lung-sparing procedure for deep metastasis, ground-glass opacities or early-stage non-small cell lung cancer (NSCLC) in selected patients, with equivalent outcome than lobectomy and well-established functional benefits (1,2).
Among complex segmentectomies, right S9+10 segmentectomy is generally considered one of the most challenging (3) and there are several reasons for that: first, the anatomy of those posterior (S10) and lateral (S9) basal segments and their adjacent superior (S6), medial (S7) and anterior (S8) segments, is highly variable; second, the broncho-vascular segmental targets are deeply located within the parenchyma, making their dissection slightly tricky; third, intersegmental plane (ISP) precise delimitation requires a complex three-dimensional stapling approach for extraction of a cuboid S9+10 double segment from a pyramidal lower lobe. Thus, preoperative three-dimensional computed tomography (3D-CT) broncho-vascular reconstruction appears nowadays essential and the use of intraoperative techniques for intersegmental plane accurate delimitation, such as fluorescence imaging, is clearly mandatory in order to tackle this complexity.
Historically, two main strategies have been proposed. One is an “interlobar fissure-first” approach involving identification of lower lobe arterial branches before exposure of the hilum of basal segments by creating a posterior tunnel, allowing S6 to be partially or totally separated from the basal segments through a posterior or a bidirectional approach (4-6). The other is a single-directional “inferior pulmonary ligament” approach, tracking the target segmental branches, starting with venous distal confluence dissection, followed by bronchial elements before segmental arterial identification, described as the “stem-branch” technique, without the need for interlobar fissure dissection (7-10).
We present here a right S9+10 segmentectomy through an original bidirectional subxiphoid approach, combining both “interlobar fissure-first” and “inferior pulmonary ligament” approaches associated with a so called “open book” diaphragmatic tunnelling technique between S9+10 and S7+8 for ISP delimitation. The procedure was assisted by preoperative 3D-CT broncho-vascular reconstruction and intraoperative fluorescence imaging. We present the following article in accordance with the CARE reporting checklist (available at https://jovs.amegroups.com/article/view/10.21037/jovs-21-49/rc).
Case presentation
A 77-year-old man with a metastatic colorectal neoplasia who underwent 2 years before a right hepatectomy for radical metastasis resection, was referred to our department. Chest CT showed 3 lung solid nodules increasing in size up to 1 cm, one deeply located on the right between S9 and S10 and two on the left, one deep seated in S8 and another more superficial in S6. Pulmonary functional tests were satisfactory without associated comorbidities and a bilateral lung-sparing strategy was decided after multidisciplinary team concertation. A right S9+10 segmentectomy was first planned, before a double left S8 segmentectomy and S6 wedge in a second phase, 1 month later.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images/video. A copy of the written consent is available for review by the editorial office of this journal.
Anatomical analysis (Figures 1-4)
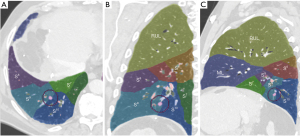
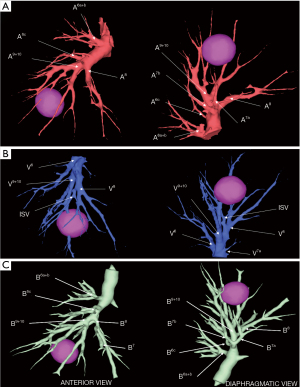
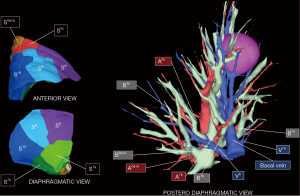
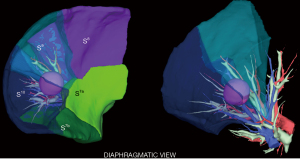
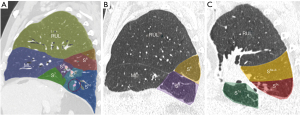
A personalized preoperative anatomical map was designed on the basis of CT 1 mm slice axial images and 3D-CT reconstruction (Fujifilm Synapse 3DTM). Careful analysis of nodule localization was confronted to multiplanar reformation segmentation (Figure 1) and positional relationships reconstruction of segmental vessel and bronchi (Figure 2) were corelated with basic anatomical landmarks as described by Nomori and Okada (11).
S9+10 broncho-vascular anatomical pattern was quite classical with common A9+10 and B9+10 trunks, whereas the Inferior basal (IBV) vein was found to be a true V9+10 without any accessory venous drainage for S8 or intersegmental plane, with superior basal vein (SBV) receiving V8 and ISV between S8 and S9.
Notably, some relevant anatomical variations (Figure 3) were observed on adjacent segments: in particular, superior S6 presented an atypical lateral S6c sub-segment separated from S6a+b, with A6c and B6c rising distally on basilar bronchial and arterial trunks; also, a protruding medial S7b extending over S10 was identified, with B7b and A7b running posteriorly between V6 and basal vein. This rare variation (14% in frequency) (12) had to be taken in consideration, since S7b subsegment resection was necessary in this case for sufficient margin and to avoid S7 congestion, as recommended by Sato and colleagues (5).
In addition, a 3D-CT reconstruction was used for simulation of a 20 mm diameter “halo” around the nodule, that was confronted to the volumetric segmentation in order to anticipate the safety reliable margins for an oncological segmentectomy (Figure 4).
Operative techniques (Video 1 and Table 1)
Table 1
| Step 1: anterior interlobar fissure approach |
| Completion of the fissure |
| Exposure of PA, A9+10 and adjacent A8 and A6c |
| Dissection and stapling of A9+10 |
| #11 and #12 node dissection |
| Step 2: posterior mediastinal dissection |
| Inferior pulmonary ligament section |
| Subcarinal lymph node dissection |
| #11 node dissection |
| Step 3: inferior pulmonary ligament approach |
| Dissection and identification of V6 and basal segmental veins |
| Dissection and stapling of B7b between V6 and basal vein |
| Distal dissection of V6 and identification of A7b |
| ISP1 diaphragmatic stapling between S7b and S7a |
| Further dissection of V8 “bookmark” |
| Dissection and clip of V9+10 |
| Dissection and clip of A7b |
| Dissection of B9+10 and identification of adjacent B6c and B8 |
| Step 4: diaphragmatic “open book” basal ISP division |
| Fluorescence imaging system use after ICG injection (0.3 mg/kg) |
| ISP delimitation by cauterisation |
| ISP2 and ISP3 anterior stapling between S8 and S9 |
| Further distal dissection of V8 and intersegmental vein “bookmark” |
| ISP4 diaphragmatic tunnel stapling between S10 and S8 |
| Dissection and stapling of B9+10 |
| Retrieval of A9+10 stump behind B9+10 stump |
| Identification of the fissural tunnel between S9+10 and S8 |
| S9+10 stump traction rearward |
| ISP5 diaphragmatic tunnel stapling between S9 and S8 |
| Step 5: superior and posterior S6 ISP division |
| Further dissection of V6a+b |
| ISP6 posterior stapling along V6 |
| Identification of A6c and B6c |
| S9+10 stump traction downward |
| ISP7 and ISP8 superior and posterior stapling between S9+10 and S6 |
PA, pulmonary artery; ISP, intersegmental plane; ICG, indocyanine green.
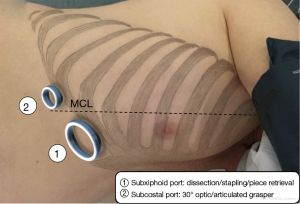
The procedure was performed under thoracoscopic 4K 30° imaging system with immersive 55-inch screen, camera holder stabilization, through a multiportal subxiphoid approach, whose principles have already been described (13). A specific biportal approach has been standardized in our department for all regular right-side resections, with one 4 cm subxiphoid utility port dedicated to dissection, stapling and specimen extraction, and another 1 cm subcostal port on the medio clavicular line used for camera and an articulated grasper (Figure 5). The surgeons stand both in the front of the patient.
Step 1: anterior interlobar fissure approach
The interlobar pulmonary artery and its branches were first exposed after completing the fissure. Then, A9+10 was encircled and stapled after clear identification of adjacent A8 and A6c arteries. In cases of hypoplastic or incomplete fissure, this approach may be tricky and we usually overcome this by the use of the fissure tunnel technique (14), which prevents postoperative airleak in the same time. This approach is also mandatory in NSCLC indications for an adequate radical hilar lymph node (LN) dissection of stations 10, 11 and 12, with a frozen section recommended.
Step 2: posterior mediastinal dissection
The patient was then rolled anteriorly and the lower lobe also flipped anteriorly, giving access to subcarinal area for a lobe specific LN dissection of stations 7, 8 and 9, after inferior pulmonary ligament section and sampling of station 11 of the second carina.
Step 3: inferior pulmonary ligament approach
A Trendelenburg patient position was adopted at this step, optimizing the diaphragmatic view. The subxiphoid way gives an interesting panoramic inferior and tangential angle of attack, offering the possibility of an accurate dissection of venous and bronchial segmental branches. IBV was first dissected: small V7b branches were coagulated, V7a was respected and V6 and SBV were further identified. As expected, B7b and A7b were recognized running posteriorly between V6 and basal vein and B7b was first dissected and stapled. An “open book” approach was then initiated: the principle is to divide S9+10 and S7+8 following V8 “bookmark” from the diaphragmatic side. It started with a first inferior stapling (ISP1) dividing S7a and S7b, respecting V7a and B7a and guided by inferior pulmonary ligament axis. This first division—usually between S7 and S10 in classical configuration—generally dramatically enhances the access to inferior hilar structures and the deployment of stem-branch technique. IBV was then confirmed to be a true V9+10 and was stapled. V8 was further deeply dissected inside the hilar structure until intersegmental venous (ISV) branch (between S8 and S9) landmark visualization. A7b was then clipped and B9+10 and both adjacent B8 and B6c were identified.
Step 4: “open book” diaphragmatic basal ISP division
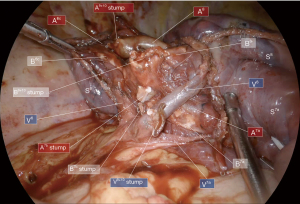
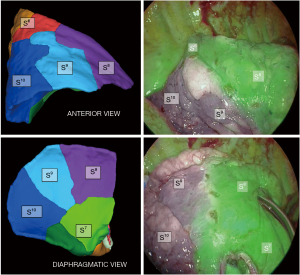
Thereafter, a near infra-red fluorescence imaging system (Medtronic ElevisionTM IR) was used with single injection of indocyanine green (ICG) at the dose of 0.3 mg/kg, and a demarcation line was clearly obtained and cauterized between S9+10 and adjacent still vascularized segments (Figure 6). The “open book” technique was deployed peripherally to centrally (Figure 7): anterior ISP stapling between S9 and S8 was continued (ISP2 & ISP3) followed by diaphragmatic tunnel stapling (ISP4) between S10 and S8, guided by the relevant V8 landmark. B9+10 appeared clearly visible at this step and was stapled. A9+10 stump was retrieved behind B9+10 stump, which was then pulled up posteriorly. The tunnel reaching previous anterior fissure dissection was identified. S9+10 stump traction is very important here in so far as it gives an optimal view for the next ISP diaphragmatic tunnel stapling (ISP5) dividing S9 and S8 centrally, from bottom to top. A small parenchymal bridge was preserved between S6 and S8, avoiding any torsion of an isolated S6.
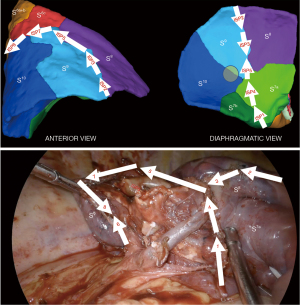
Step 5: posterior and superior S6 ISP division
The posterior and superior ISP division was finally completed between S9+10 and S6, guided by V6 posteriorly (ISP6) and by fluorescence demarcation line superiorly (ISP7 and ISP8) with a particular attention paid to A6c and B6c rising unusually distally in this case. S9+10 stump traction downward is quite helpful during this step.
Step 6: pathological examination
The piece was extracted through the subxiphoid port and metastatic nature of the nodule was confirmed on frozen section, with a sufficient 2 cm safety margin.
Step 7: final anatomical view
The final anatomical and ISP views were checked (Figures 7,8) and satisfying reventilation of remaining segments was also confirmed without airleak. A 24 Fr subxiphoid chest tube was then placed.
Postoperative course
The patient was extubated in operating room and integrated in an enhanced recovery after surgery (ERAS) programme associating early physiotherapy, feeding and mobilization and opioid-free analgesia limited to a combination of non-steroid anti-inflammatory drugs and paracetamol. He was discharged on postoperative day-1 after early chest tube removal (15) with an uneventful postoperative course. The final pathological examination confirmed complete resection of a 10 mm metastatic nodule with 2 cm margin free and 12 negative nodes. A double left S8 segmentectomy and S6 wedge was planned one month later after CT control, showing a quite satisfying ventilation of remaining adjacent segments (Figure 9).
Discussion
Thoracoscopic anatomical segmentectomy is actually a standard of care for surgical treatment of deep metastasis and ground glass opacities and is taking an increasingly important place in the area of early-stage NSCLC treatment, that could be soon reinforced by the expected long-term results of the major ongoing JCOG-0802 study on the topics (16). If we add this to the trend in emergence of efficient lung cancer screening programs (17), thoracic surgeons will certainly have to deal in the near future with a wide and growing range of complex segmentectomy of which right S9+10 segmentectomy is probably one of the most challenging.
Three main issues can be highlighted to explain this complexity, one around the high frequency of anatomical variation, another concerning the deep-seated location of broncho-vascular segmental targets and last but not least the multi-directional three-dimensional stapled-based delimitation of intersegmental plane. Overcoming these 3 key points requires above all an overall personalized planning strategy, involving imperative anatomical preoperative 3D-CT planning for an accurate anatomical knowledge, preoperative technical planning for an appropriate tracking of broncho-vascular segmental elements and again preoperative planning of ISP delimitation for which fluorescence imaging is becoming the technique of choice.
We described herein an original subxiphoid approach for achieving such complex segmentectomy, combining both interlobar fissure and inferior pulmonary ligament approaches. Although still little used, subxiphoid approach has already proven to be feasible, safe and efficient for simple as well as complex segmentectomies in experienced centers (18) and presents some advantages: it’s a highly minimally invasive painless approach offering a great synergy with ERAS programmes and giving a particularly interesting view for basal segmentectomies. The standardized subxiphoid biportal approach, which was developed in our department for right-side resection (14), supports this statement: the duplicated subcostal panoramic view allows to deal with the same vision quality whether for interlobar fissure or inferior pulmonary ligament step, while leaving a great ease of movement and dissection accuracy through the subxiphoid port. This approach is also very interesting for deployment of tunnel techniques from anterior to posterior on fissural side in cases of hypoplastic fissure (15) as from bottom to top on diaphragmatic side for ISP delimitation.
When focusing on technical points, some authors recommend to start with interlobar fissure arterial dissection with a view to initiate the posterior tunnel giving the way of ISP between S6 and basal segments (4-6). We emphasize here that early A9+10 stapling during this first interlobar step is greatly helpful to take the better benefit of further fluorescence imaging and that splitting S6 can be delayed at the last step after the “open book” basal splitting of S9+10 from S7+8. Anterior fissure dissection also gives an easier access to hilar and segmental LN dissection, a mandatory step in NSCLC indications. Furthermore, it facilitates for us the following Inferior pulmonary ligament approach, described by its defenders as a “stem branch technique” contributing to an easier reliable tracking of deeply located targeted segmental branches, successively, venous, bronchial, and then arterial one (7-10). This unidirectional inferior approach avoids also unnecessary fissure dissection and airleaks that goes with it but is not totally satisfactory in an oncological perspective. We decided then to associate the advantages of both approaches through this original bidirectional subxiphoid way.
ISP management also remains a major issue: bronchial inflation method was historically the first used. It is now regarded as too approximate due to the frequency of residual parenchymal bridges between segments, particularly in patients with lung obstructive disorder, compared to fluorescence imaging, which is nowadays the technique of choice by its accuracy to distinguish targeted segments from adjacent still vascularized ones (19,20). Other techniques have been proposed, like slip knot method (21), endobronchial dye injection (22) and Oizumi and colleagues still consider extensive venous dissection of intersegmental veins as an important landmark for an adequate ISP delimitation (23). De facto, our “open book” diaphragmatic approach illustrates in this case the effective combination of both fluorescence imaging and ISV extensive dissection: basal ISP stapling was deployed peripherally to centrally from diaphragmatic side first, following ICG marking and V8/ISV “bookmark” before completing S6 ISP. We have to highlight here the great subxiphoid view provided for crucial S9+10 stump traction downward and rearward during this phase.
Lastly, regarding S7 adjacent segment management, described by Sato and colleagues as an often-neglected step (5), subxiphoid approach was very appropriate in conjunction with preoperative 3D-CT reconstruction, to avoid any misidentification of the S7b anatomical variation encountered in this case.
Conclusions
Subxiphoid approach is an effective alternative approach for such complex right S9+10 anatomical segmentectomy. Beyond the surgical approach chosen, complex thoracoscopic segmentectomy has nowadays to be tackled via an overall personalized planning strategy, assisted by 3D-CT anatomical reconstruction and intersegmental plane accurate delimitation techniques as fluorescence imaging and integrated in ERAS dynamics, for an optimal oncological lung-sparing sub-lobar resection.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Michel Gonzalez) for the series “VATS Segmentectomy” published in Journal of Visualized Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jovs.amegroups.com/article/view/10.21037/jovs-21-49/rc
Peer Review File: Available at https://jovs.amegroups.com/article/view/10.21037/jovs-21-49/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.com/article/view/10.21037/jovs-21-49/coif). The series “VATS Segmentectomy” was commissioned by the editorial office without any funding or sponsorship. KP declares as educational webinar lecturer for MEDTRONIC. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images/video. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Handa Y, Tsutani Y, Mimae T, et al. Surgical Procedure Selection for Stage I Lung Cancer: Complex Segmentectomy versus Wedge Resection. Clin Lung Cancer 2021;22:e224-33. [Crossref] [PubMed]
- Bédat B, Abdelnour-Berchtold E, Krueger T, et al. Impact of complex segmentectomies by video-assisted thoracic surgery on peri-operative outcomes. J Thorac Dis 2019;11:4109-18. [Crossref] [PubMed]
- Handa Y, Tsutani Y, Mimae T, et al. Complex segmentectomy in the treatment of stage IA non-small-cell lung cancer. Eur J Cardiothorac Surg 2020;57:114-21. [Crossref] [PubMed]
- Igai H, Kamiyoshihara M, Kawatani N, et al. Thoracoscopic lateral and posterior basal (S9 + 10) segmentectomy using intersegmental tunnelling. Eur J Cardiothorac Surg 2017;51:790-1. [PubMed]
- Sato M, Murayama T, Nakajima J. Thoracoscopic stapler-based "bidirectional" segmentectomy for posterior basal segment (S10) and its variants. J Thorac Dis 2018;10:S1179-86. [Crossref] [PubMed]
- Gossot D, Seguin-Givelet A. Thoracoscopic right S9+10 segmentectomy. J Vis Surg. 2018;4:181. [Crossref]
- Kikkawa T, Kanzaki M, Isaka T, et al. Complete thoracoscopic S9 or S10 segmentectomy through a pulmonary ligament approach. J Thorac Cardiovasc Surg 2015;149:937-9. [Crossref] [PubMed]
- Oizumi H, Kato H, Suzuki J, et al. Thoracoscopic anatomical S10 segmentectomy: a posterior approach. J Vis Surg 2018;4:142. [Crossref]
- Zhu Y, Pu Q, Liu L. Trans-inferior-pulmonary-ligament VATS basal segmentectomy: application of single-direction strategy in segmentectomy of left S9+10. J Thorac Dis 2018;10:6266-8. [Crossref] [PubMed]
- Liu C, Liao H, Guo C, et al. Single-direction thoracoscopic basal segmentectomy. J Thorac Cardiovasc Surg 2020;160:1586-94. [Crossref] [PubMed]
- Nomori H, Okada M. Illustrated anatomical segmentectomy for lung cancer. Springer, 2012:92-113.
- Shimizu K, Nagashima T, Yajima T, et al. Thoracoscopic Medial-Basal Segment Segmentectomy. Ann Thorac Surg 2017;104:e403-6. [Crossref] [PubMed]
- Pfeuty K, Lenot B. Multiportal subxiphoid thoracoscopic major pulmonary resections. J Thorac Dis 2019;11:2778-87. [Crossref] [PubMed]
- Decaluwe H, Sokolow Y, Deryck F, et al. Thoracoscopic tunnel technique for anatomical lung resections: a 'fissure first, hilum last' approach with staplers in the fissureless patient. Interact Cardiovasc Thorac Surg 2015;21:2-7. [Crossref] [PubMed]
- Pfeuty K, Lenot B. Early postoperative day 0 chest tube removal using a digital drainage device protocol after thoracoscopic major pulmonary resection. Interact Cardiovasc Thorac Surg 2020;31:657-63. [Crossref] [PubMed]
- Suzuki K, Saji H, Aokage K, et al. Comparison of pulmonary segmentectomy and lobectomy: Safety results of a randomized trial. J Thorac Cardiovasc Surg 2019;158:895-907. [Crossref] [PubMed]
- de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N Engl J Med 2020;382:503-13. [Crossref] [PubMed]
- Ali J, Haiyang F, Aresu G, et al. Uniportal Subxiphoid Video-Assisted Thoracoscopic Anatomical Segmentectomy: Technique and Results. Ann Thorac Surg 2018;106:1519-24. [Crossref] [PubMed]
- Yotsukura M, Okubo Y, Yoshida Y, et al. Indocyanine green imaging for pulmonary segmentectomy. JTCVS Tech 2021;6:151-8. [Crossref] [PubMed]
- Guigard S, Triponez F, Bédat B, et al. Usefulness of near-infrared angiography for identifying the intersegmental plane and vascular supply during video-assisted thoracoscopic segmentectomy. Interact Cardiovasc Thorac Surg 2017;25:703-9. [Crossref] [PubMed]
- Oizumi H, Kato H, Endoh M, et al. Slip knot bronchial ligation method for thoracoscopic lung segmentectomy. Ann Thorac Surg 2014;97:1456-8. [Crossref] [PubMed]
- Sato M, Omasa M, Chen F, et al. Use of virtual assisted lung mapping (VAL-MAP), a bronchoscopic multispot dye-marking technique using virtual images, for precise navigation of thoracoscopic sublobar lung resection. J Thorac Cardiovasc Surg 2014;147:1813-9. [Crossref] [PubMed]
- Oizumi H, Kanauchi N, Kato H, et al. Anatomic thoracoscopic pulmonary segmentectomy under 3-dimensional multidetector computed tomography simulation: a report of 52 consecutive cases. J Thorac Cardiovasc Surg 2011;141:678-82. [Crossref] [PubMed]
Cite this article as: Rojas D, Lenot B, Pfeuty K. Thoracoscopic subxiphoid right S9+10 segmentectomy: a case report. J Vis Surg 2023;9:19.