
How to identify the intersegmental plane?—a narrative review
Introduction
Lobectomy is currently the gold standard treatment of non-small cell lung cancer (NSCLC) (1). However, segmentectomy is a parenchyma-sparing procedure that is considered as effective as lobectomy in the treatment for early-stage NSCLC (stage IA1 and IA2) (2). In parallel, the video-assisted thoracic surgery (VATS) technique is associated with less postoperative pain and a better quality of life as compared to an open approach for pulmonary anatomical resection (3). Consequently, segmentectomy by VATS is being used increasingly. However, the difficulty in identifying the intersegmental plane (ISP) may remain a technical barrier during the procedure. A 3-dimensional pre-operative planning provides a better understanding of the anatomy (4,5). Nevertheless, the surgeon needs intraoperative assistance to identify the ISP correctly. Furthermore, according to the American College of Chest Physicians, safety margins of 2 cm for tumors larger than 2 cm or a margin that is at least as large as the tumour diameter for smaller lesions are required to avoid local recurrence (6,7). Therefore, an adequate identification of the ISP during segmentectomy is of special concern for the oncological outcome and survival. Several technical procedures have been proposed to identify the ISP during VATS segmentectomy.
The aim of this narrative review is to describe the present state of methods to identify the ISP. We present the following article in accordance with the Narrative Review reporting checklist (available at https://jovs.amegroups.com/article/view/10.21037/jovs-21-40/rc).
Methods
This narrative review was based on searches in MEDLINE, PubMed and Google Scholar database. Keywords used for the research included “segmentectomy by VATS”, “thoracoscopic segmentectomy”, “ISP” and “intersegmental demarcation”. We searched for published articles between 2000 and 2021. The articles were reviewed by B.B and W.K. Duplicate articles were removed. Studies with content unrelated to the topic, videos, animal studies, articles not written in English and studies prior 2000 were excluded from this narrative review.
Discussion
Identification of the ISP is a key step in segmentectomy by VATS. Many new techniques are constantly emerging that are driven by new advances in technology. Currently, no demarcation technique of the ISP has really succeeded in establishing itself universally and each technique has its own advantages and disadvantages. Considering the anatomy of a pulmonary segment, the pulmonary artery and bronchus generally follow a similar path. The vein is often located in the ISP. Lymphatic drainage follows the bronchi and pulmonary veins. Classically, the ISP is determined through the segmental artery and bronchus and its visualization is obtained at the visceral pleura. A recent clinical trial showed that the dissection of the ISP with stapling devices improved the postoperative outcome as compared to dissection with electrocautery (8). The intra-parenchymatous ISP is also often not determined during segmentectomy by VATS.
The identification of the ISP is established through the segmental bronchus and/or the segmental artery (Table 1). In addition, different dyes can be injected into the bronchus or the artery and different gases can be used to ventilate the target segmental bronchus. The target segment may be marked positively or negatively. Moreover, intraoperative augmented reality can aid to determine the ISP. The different methods are summarized below.
Table 1
| ISP demarcation | Techniques | Pros | Cons |
|---|---|---|---|
| Bronchial demarcation | Inflation-deflation | Easy | Imprecision (collateral ventilation) |
| Inexpensive | Reduce the thoracic working space | ||
| Time consuming in emphysematous patients (entrapped air) | |||
| Modified inflation-deflation method | Easy | Imprecision (collateral ventilation) | |
| Inexpensive | Time consuming | ||
| Selective inflation of the target segment | Inexpensive | Need for skilled staff | |
| Affect little the thoracic working space | Imprecision (collateral ventilation) | ||
| Visual control of the target segment during bronchoscopy | |||
| Intrabronchial injection of dyes | No need of inflation | Need for skilled staff | |
| Can mark the resection margins (VAL-MAP) | Inadequate demarcation if ICG leaks backward | ||
| Cost | |||
| Arterial demarcation | ICG | Easy | Cost |
| Quick | |||
| Repeatable | |||
| No need of inflation | Imprecision in emphysematous patients or collateral circulation | ||
| Accurate detection of ISP | |||
| Could increase the resection margin length | |||
| Hyperspectral imaging | Same advantages as ICG | Cost | |
| No need of intravenous injection |
ISP, intersegmental plane; ICG, indocyanine green; VAL-MAP, virtual-assisted lung mapping.
Bronchial demarcation
Inflation-deflation
The inflation-deflation method is the historically manoeuver used to establish the ISP in open procedure (9,10). This technique consists of occluding the target segmental bronchus and then to inflate the lung. This technique creates a demarcation line between the deflation of the segment to be resected and the inflation of the remaining lung. However, the determination of the border can be difficult due to collateral ventilation through the pores of Kohn and the canals of Lambert into an adjacent segment. In VATS, this method limits the thoracic working space, especially in emphysematous lungs. Furthermore, this method can be time consuming in emphysematous patients due to entrapped air into the alveoli and the difficulty to empty their lung after selective exclusion.
Modified inflation-deflation method
In 2000, Tsubota described the opposite of the inflation-deflation method. The lobe is inflated after the identification of the segmental bronchus and the dissection of the artery and the intersegmental vein. The bronchus is then dissected to maintain gas inside and thereafter the lung is deflated (11). After 10–15 min, the ISP is demarcated. Interestingly, demarcation of the ISP with the modified inflation-deflation method seems also possible with the dissection of the arteria alone, with comparable results than after bronchial dissection (12). This method should coincide with a hybrid vascular and bronchial approach. The modified inflation-deflation method is inexpensive but is time-consuming and collateral ventilation or airway secretions could impeded the identification of the ISP. A recent clinical trial showed that the use of nitrous oxide/oxygen inspired mixture during the modified inflation-deflation method decreased the time to identify the ISP from 16 to 5 min (13). Chang et al. added an intraoperative computed tomography navigation after an inflation-deflation method in a hybrid operating room. This interesting technique permitted to control the resection margin (14).
Selective inflation of the target segment
Contrary to the conventional inflation-deflation method, the target segment is here directly inflated. After isolation of the targeted segmental bronchus by VATS, a high-frequency jet ventilation (40 Hz, working pressure of 2 kg/cm2) is injected in the target segment by bronchoscopy (15). The selected bronchus is then ligated and inflated while the preserved segments are deflated. This method affects little on the visual field during VATS but requires skilled staff. Nevertheless, the issue of collateral ventilation can remain. Another proposed method for a selective inflation is the puncture of the bronchus with a needle by the surgeon (16). However, we do not recommend this method because of the risk of massive air embolism (17).
Intrabronchial injection of dyes
In 2012, Sekine et al. reported the use of an infrared thoracoscopy after preoperative or intraoperative transbronchial injection of indocyanine green (ICG) (18). ICG is a green dye visible as fluorescence by near-infrared light. The target segment appears as a bright area. With a bronchoscope, 20 to 30 mL of 5-fold saline-diluted ICG is injected into each target segmental bronchus. Then, 200 to 300 mL of air was injected into the bronchus to distribute ICG into peripheral regions. A continuous positive airway pressure ventilation was maintained until the start of the operation. In a recent study, determination of the optimal settings was developed (19). They established precisely that the optimal volume of diluted ICG for insufflation was 8.91% of the calculated targeted pulmonary segment volume. This technique no need for lung inflation. However, successful identification of the ISP depends on skilled staff, and airway secretions may interfere with staining. Furthermore, an inadequate demarcation of the ISP can occur if some of the insufflated ICG leaks backward. In order to overcome this issue, a Fogarty arterial embolectomy catheter may be used for sealing the bronchial lumen (20).
To improve the localization of the nodule during VATS, an electromagnetic navigation bronchoscope (ENB) can be used. This repeated procedure with multi-spot dye-marking to select the target segment is called a virtual-assisted lung mapping (VAL-MAP) (21). This method was originally developed as an alternative to CT-guided marking. This technique has some advantages for VATS segmentectomy: the resection margins can be marked and the resection lines can be flexibly designed in another adjacent segment. In addition to the VAL-MAP, bronchoscopic placement of platinum microcoils can be placed to indicate appropriate resection margins (22).
Arterial demarcation
In 2009, Misaki et al. first reported injecting ICG intravenously intraoperatively to identify the ISP after resection of the segmental artery (23). Since then, this method has been widely studied (24-31). This method is the one we use. Contrary to the intrabronchial injection method, the target segment is dark while the remaining lung appears bright (Figure 1). After division of the target artery, ICG is injected into the central or peripheral vein with an initial dose of bolus of 0.15 mg/kg. The ISP in then marked with electrocautery. Intravenous ICG method has major advantages: it’s very quick, repeatable multiple times, there is no need for a skilled staff, and it permits to verify if the target segment is a reasonable consideration for resection by clamping the artery. In a previous study, we considered that intravenous ICG changed the arterial dissection in 10% of patients, avoiding inappropriate resection (31). Furthermore, Mehta et al. showed that the use of ICG during segmentectomy could increase the oncological margin length as compared to the best judgement of the surgeon (32). However, the ISP may be misleading or confusing due to emphysema or collateral circulation.
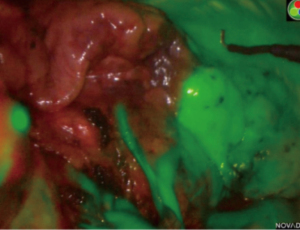
Sun et al. compared the intravenous ICG method with the conventional inflation-deflation method. Both methods were used in 19 patients to delineate the ISP (33). They found that intravenous ICG method was totally concordant with the modified inflation-deflation method. Finally, the benefit of a hybrid technique of VAL-MAP and systemic injection was recently demonstrated for a nodule deeply located between the left upper division segment and the left lingular segment (34).
Conclusions
Hybrid procedures with mixing techniques have generated a plethora of articles on the feasibility of each procedure. All techniques seem to be associated with a high success rate but only one study compared different methods in the same patients (33). Finally, the last method not published but practiced is the identification of the ISP by the eyes of the surgeons. Another promising system to identify ISP during VATS is the hyperspectral imaging that would permit to display tissue oxygen saturation and other parameters without dye. Frankly, none of these methods has shown its oncological superiority over another. Margin resection in segmentectomy is a matter of concern to avoid local recurrence. Segmentectomy can only be indicated and performed if the resection can be safe and oncological. The best technique to identify the ISP is ultimately the one that can avoid recurrence.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Visualized Surgery for the series “VATS Segmentectomy”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jovs.amegroups.com/article/view/10.21037/jovs-21-40/rc
Peer Review File: Available at https://jovs.amegroups.com/article/view/10.21037/jovs-21-40/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.com/article/view/10.21037/jovs-21-40/coif). The series “VATS Segmentectomy” was commissioned by the editorial office without any funding or sponsorship. MG served as the unpaid Guest Editor of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Zeng W, Zhang W, Zhang J, et al. Systematic review and meta-analysis of video-assisted thoracoscopic surgery segmentectomy versus lobectomy for stage I non-small cell lung cancer. World J Surg Oncol 2020;18:44. [Crossref] [PubMed]
- Bendixen M, Jørgensen OD, Kronborg C, et al. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836-44. [Crossref] [PubMed]
- Yang Q, Xie B, Hu M, et al. Thoracoscopic anatomic pulmonary segmentectomy: a 3-dimensional guided imaging system for lung operations. Interact Cardiovasc Thorac Surg 2016;23:183-9. [Crossref] [PubMed]
- Sarsam M, Glorion M, de Wolf J, et al. The role of three-dimensional reconstructions in understanding the intersegmental plane: an anatomical study of segment 6. Eur J Cardiothorac Surg 2020;58:763-7. [Crossref] [PubMed]
- Schuchert MJ, Pettiford BL, Keeley S, et al. Anatomic segmentectomy in the treatment of stage I non-small cell lung cancer. Ann Thorac Surg 2007;84:926-32; discussion 932-3. [Crossref] [PubMed]
- Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e278S-e313S.
- Chen X, Jin R, Xiang J, et al. Methods for Dissecting Intersegmental Planes in Segmentectomy: A Randomized Controlled Trial. Ann Thorac Surg 2020;110:258-64. [Crossref] [PubMed]
- OVERHOLT RH. WOODS FM, BETTS RH. An improved method of resection of pulmonary segments; report of a technique applied in 70 operations. J Thorac Surg 1948;17:464-79. [Crossref] [PubMed]
- RUBENSTEIN LH. O'NEILL TJ, GLOVER RP. A technique for pulmonary segmental delineation. J Thorac Surg 1949;18:75-81. [Crossref] [PubMed]
- Tsubota N. An improved method for distinguishing the intersegmental plane of the lung. Surg Today 2000;30:963-4. [Crossref] [PubMed]
- Fu HH, Feng Z, Li M, et al. The arterial-ligation-alone method for identifying the intersegmental plane during thoracoscopic anatomic segmentectomy. J Thorac Dis 2020;12:2343-51. [Crossref] [PubMed]
- Yang W, Liu Z, Yang C, et al. Combination of nitrous oxide and the modified inflation-deflation method for identifying the intersegmental plane in segmentectomy: A randomized controlled trial. Thorac Cancer 2021;12:1398-406. [Crossref] [PubMed]
- Chang SS, Okamoto T, Tokunaga Y, et al. Intraoperative Computed Tomography Navigation During Thoracoscopic Segmentectomy for Small-sized Lung Tumors. Semin Thorac Cardiovasc Surg 2018;30:96-101. [Crossref] [PubMed]
- Okada M, Mimura T, Ikegaki J, et al. A novel video-assisted anatomic segmentectomy technique: selective segmental inflation via bronchofiberoptic jet followed by cautery cutting. J Thorac Cardiovasc Surg 2007;133:753-8. [Crossref] [PubMed]
- Kamiyoshihara M, Kakegawa S, Ibe T, et al. Butterfly-needle video-assisted thoracoscopic segmentectomy: a retrospective review and technique in detail. Innovations (Phila) 2009;4:326-30. [Crossref] [PubMed]
- Otsuka T, Nakamura Y, Harada A, et al. Extremely rare but potential complication of diffuse brain edema due to air embolism during lung segmentectomy with selected segmental inflation technique by syringe needle during video-assisted thoracoscopic surgery. J Thorac Cardiovasc Surg 2011;142:e151-2. [Crossref] [PubMed]
- Sekine Y, Ko E, Oishi H, et al. A simple and effective technique for identification of intersegmental planes by infrared thoracoscopy after transbronchial injection of indocyanine green. J Thorac Cardiovasc Surg 2012;143:1330-5. [Crossref] [PubMed]
- Anayama T, Hirohashi K, Miyazaki R, et al. Fluorescence visualization of the intersegmental plane by bronchoscopic instillation of indocyanine green into the targeted segmental bronchus: determination of the optimal settings. J Int Med Res 2021;49:300060521990202. [Crossref] [PubMed]
- Sekine Y, Itoh T, Toyoda T, et al. Precise Anatomical Sublobar Resection Using a 3D Medical Image Analyzer and Fluorescence-Guided Surgery With Transbronchial Instillation of Indocyanine Green. Semin Thorac Cardiovasc Surg 2019;31:595-602. [Crossref] [PubMed]
- Sato M, Omasa M, Chen F, et al. Use of virtual assisted lung mapping (VAL-MAP), a bronchoscopic multispot dye-marking technique using virtual images, for precise navigation of thoracoscopic sublobar lung resection. J Thorac Cardiovasc Surg 2014;147:1813-9. [Crossref] [PubMed]
- Sato M, Nagayama K, Kobayashi M, et al. Virtual-Assisted Lung Mapping 2.0: Preoperative Bronchoscopic Three-Dimensional Lung Mapping. Ann Thorac Surg 2019;108:269-73. [Crossref] [PubMed]
- Misaki N, Chang SS, Gotoh M, et al. A novel method for determining adjacent lung segments with infrared thoracoscopy. J Thorac Cardiovasc Surg 2009;138:613-8. [Crossref] [PubMed]
- Kasai Y, Tarumi S, Chang SS, et al. Clinical trial of new methods for identifying lung intersegmental borders using infrared thoracoscopy with indocyanine green: comparative analysis of 2- and 1-wavelength methods. Eur J Cardiothorac Surg 2013;44:1103-7. [Crossref] [PubMed]
- Iizuka S, Kuroda H, Yoshimura K, et al. Predictors of indocyanine green visualization during fluorescence imaging for segmental plane formation in thoracoscopic anatomical segmentectomy. J Thorac Dis 2016;8:985-91. [Crossref] [PubMed]
- Kuroda H, Yoshida T, Arimura T, et al. Novel development of Spectra-A using indocyanine green for segmental boundary visibility in thoracoscopic segmentectomy. J Surg Res 2018;227:228-33. [Crossref] [PubMed]
- Tarumi S, Misaki N, Kasai Y, et al. Clinical trial of video-assisted thoracoscopic segmentectomy using infrared thoracoscopy with indocyanine green. Eur J Cardiothorac Surg 2014;46:112-5. [Crossref] [PubMed]
- Guigard S, Triponez F, Bédat B, et al. Usefulness of near-infrared angiography for identifying the intersegmental plane and vascular supply during video-assisted thoracoscopic segmentectomy. Interact Cardiovasc Thorac Surg 2017;25:703-9. [Crossref] [PubMed]
- Mun M, Okumura S, Nakao M, et al. Indocyanine green fluorescence-navigated thoracoscopic anatomical segmentectomy. J Vis Surg 2017;3:80. [Crossref] [PubMed]
- Pischik VG, Kovalenko A. The role of indocyanine green fluorescence for intersegmental plane identification during video-assisted thoracoscopic surgery segmentectomies. J Thorac Dis 2018;10:S3704-11. [Crossref] [PubMed]
- Bédat B, Triponez F, Sadowski SM, et al. Impact of near-infrared angiography on the quality of anatomical resection during video-assisted thoracic surgery segmentectomy. J Thorac Dis 2018;10:S1229-34. [Crossref] [PubMed]
- Mehta M, Patel YS, Yasufuku K, et al. Near-infrared mapping with indocyanine green is associated with an increase in oncological margin length in minimally invasive segmentectomy. J Thorac Cardiovasc Surg 2019;157:2029-35. [Crossref] [PubMed]
- Sun Y, Zhang Q, Wang Z, et al. Is the near-infrared fluorescence imaging with intravenous indocyanine green method for identifying the intersegmental plane concordant with the modified inflation-deflation method in lung segmentectomy? Thorac Cancer 2019;10:2013-21. [Crossref] [PubMed]
- Yanagiya M, Hiyama N, Matsumoto J. Hybrid technique of virtual-assisted lung mapping and systemic indocyanine green injection for extended segmentectomy. Surg Case Rep 2020;6:273. [Crossref] [PubMed]
Cite this article as: Bédat B, Gonzalez M, Karenovics W. How to identify the intersegmental plane?—a narrative review. J Vis Surg 2022;8:37.


