Complex mitral valve regurgitation: surgical evaluation, approach and repair techniques
Introduction
Degenerative mitral valve disease is the most prevalent valve dysfunction in the developed world (1). Mucoid degeneration often affects more than one element of the mitral apparatus, ultimately leading to incompetence. Surgical reconstruction aims to restore valve competence using a functional rather than a geometric repair approach. Although various strategies have been shown effective over the past half of the century, the wide variability in the techniques, surgical approaches and strategies have prevented attempts to standardize surgical therapy, which thus remains a case of expert opinion and individual preference.
The present review focuses on the principal tenets of our operative strategy for the surgical repair of degenerative mitral valve disease.
Basic principles
Degenerative valve disease is a process both dynamic and progressive, meaning that the central hemodynamic abnormality (valve regurgitation and incremental increase in preload) signals further valve remodeling, the emergence of new lesions and the progression of the disease (MR begets MR) until such time when ventricular compensation is surpassed and chamber dilatation heralds the onset of symptoms. As such, early repair should ensure the timely interruption of this precipitous process before the advent of symptoms, and long-term durability to effectively mitigate the risk of recurrent MR. To achieve a favorable outcome, the following principles should be applied regardless of preference in operative strategy: (I) target all areas of pathology; (II) correct excess leaflet height; (III) normalize leaflet volume; (IV) address any clefts or indentations; (V) choose an appropriate annuloplasty device and (VI) blend techniques to tailor a strategy specific to the lesions encountered (2-4).
Pathophysiologic triad
A simple yet effective method to approach valve dysfunction in the context of operative planning, devised by Alain Carpentier, is Etiology → Lesion(s) → Dysfunction (5); in other words, etiology is the cause of the valve disease (organic/primary versus functional/secondary), lesions result from the specific disease etiology and, dysfunction results from the lesions.
Valve analysis
With the recent advancements in the image quality derived from transthoracic echocardiography, including real-time 3-dimensional volume rendering, the role of transesophageal echocardiography (TEE) has been more confined to the perioperative management or, in rare cases, when TTE analysis is insufficient in clarifying the pathoanatomy of the valve (6). In our mitral reference center, we rarely request preoperative TEE when good quality TTE is available in degenerative mitral valve patients.
Surgical strategy
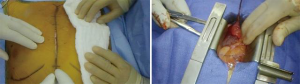
The attached intraoperative video demonstrates our surgical strategy for complex Barlow’s cases. We perform all first-time mitral repairs using a small-access approach (Figure 1) via a ~3 in × 3 in (8 cm × 8 cm) sternotomy access (Figure 2), using antegrade and retrograde blood cardioplegia for myocardial protection.
Operative approach
Where to start
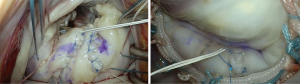
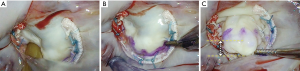
The following structured approach is a useful aid to translate valve analysis into repair strategy: as a first step, intraoperative assessment should aim to identify and confirm all the lesions seen on the preoperative and on-table echocardiogram; annuloplasty sutures are placed next, to improve valve exposure, followed by a secondary valve survey to determine any additional lesions not appreciated on preoperative imaging. Although control of leaflet height, volume and support are the main targets of repair, clefts, indentations and commissural pathology should also be addressed individually to ensure repair durability. In bileaflet disease, the posterior leaflet should be addressed first, followed by the anterior leaflet. Of note, timely identification of potential risk factors for systolic anterior motion (SAM) should always be considered during preoperative assessment, as it may impact surgical strategy. We take our time to perfect the repair and optimize the coaptation zone by pressurizing the left ventricle (saline-test) and marking the leaflet closure line (ink-test) to ensure that at least 8 mm of coaptation surface depth is below the ink mark line (Figure 3).
Leaflet resection: tailored approach
Resection strategies aim to correct excess height and volume in target areas of pathology while avoiding undue tension of the reconstructed leaflet. As a rule, we increasingly favor a more “respect” approach to leaflet tissue, with targeted resection limited to the segment with the most pathologic stigmata, followed by a tension-free leaflet reconstruction. Adjacent clefts and indentations can be partially closed via free-edge remodeling.
Leaflet height/volume management
Triangular resection
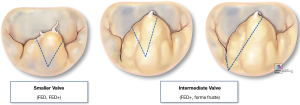
Triangular resection is a versatile technique that can adapt to correct leaflet height and/or volume by simply changing the height and/or angle of the two triangle’s sides with the triangle’s base oriented toward the leaflet’s free margin (Figure 4):
- Small triangle: symmetric (Figure 5);
- Larger triangle: symmetric (Figure 6);
- Larger triangle: asymmetric (Figure 7).
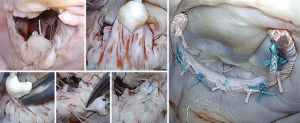
Quadrangular resection
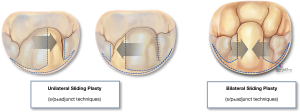
Quadrangular resection is an effective strategy to reduce both leaflet volume and height when excess tissue involves all segments of the posterior leaflet or both leaflets (as seen with Barlow’s and forme fruste). Originally devised by Alain Carpentier, quadrangular resection takes advantage of all the available leaflet surface area by removing part of the leaflet (from free edge to annular hinge), and detaching either one or both remaining segments on either side of the resection along their annular insertion to appropriate/advance the resection margins and reconstruct the leaflet (Figure 8). Alternatively, when the available leaflet tissue is not sufficient (hypoplastic, calcification, endocarditis etc.) annular plication may be a useful strategy to achieve a tension-free reconstruction by reducing the resection gap (reducing the annular perimeter). In our mitral reference center, we have increasingly adopted a more conservative approach to leaflet resection, favoring the use of smaller, targeted asymmetric triangles combined with sliding leaflet plasty which allows for effective control of leaflet size while also maintaining optimal coaptation depth following reconstruction (Video 1).
Chordal management
Leaflet support is an integral element of every reconstructive strategy and requires careful assessment during valve analysis. The main purpose of chordal management is two-fold: (I) to support poor-quality primary chordae (i.e., FED) and (II) ventricular leaflet displacement to prevent SAM. Native chordae integrity and height (or length) can help guide the margins of leaflet resection (often defined as the point on the leaflet’s free edge with healthy chordae just adjacent to the affected segment), and to identify the physiologic plane of leaflet closure.
Chordal replacement
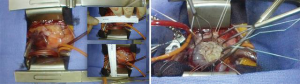
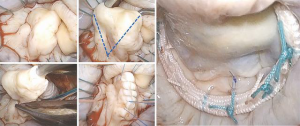
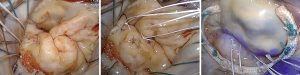
Artificial chordae from interweaved fibers of expanded polytetrafluoroethylene (ePTFE) can offer new support, reinforce the existing native chordal support and/or displace the target segment further in the ventricle to ensure a deep posterior coaptation depth during valve closure. This method may be used as an adjunct (Figure 9) or as the primary repair strategy in non-resection repair techniques (Figure 10).
Chordal transfer
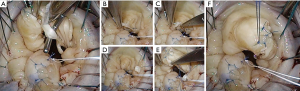
It is mostly reserved for anterior leaflet prolapse, whereby a healthy secondary chord is divided at its leaflet insertion point and re-inserted (transferred) to the free margin of the segment being repaired. It may be reinforced with an additional artificial chord, implanted at the same papillary tip with the transferred native chord (Figure 11).
Chordal division
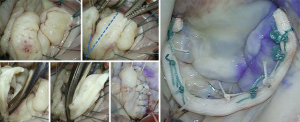
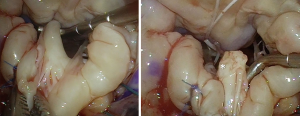
A lesion often encountered in complex advanced Barlow’s disease is the fibrotic thickening and/or fusion of one or more chordae (Figure 12), leading to retraction and subsequent tethering of the attached segment(s). It is also seen in hypoplastic leaflet segments, or due to calcific degeneration, chronic atrial fibrillation or an infectious process. In such cases, division of the restrictive chordae is necessary where possible, and mobilization of the leaflet body to assess the extent in which the undermined segment can contribute to the coaptation or whether it should be included in the primary repair strategy (Figure 13). Chordal division is also part of sliding leaflet plasty whereby secondary and tertiary chordae from the detached leaflet are divided to avoid tethering and undue tension after the segment is mobilized medially during leaflet advancement.

Clefts and indentations
As leaflet volume expands normal clefts and indentations may appear to increase in depth and/or prolapse or become restricted depending on the associated lesions and involvement of their chordae support. An effective method to address these lesions is based on (I) their proximity to the primary target of repair (i.e., the prolapsed leaflet) and (II) the availability of pliable leaflet tissue. When deep clefts are found at either side of the main prolapsing segment, they can be either sutured creating a single segment to address with a resection technique, or become one of the resection margin, the other taken at the tallest point of the leaflet’s body, followed by reconstruction. When a deep cleft is far from the planned repair, it can be closed primarily provided suturing doesn’t create tension or restriction. If primary closure is likely to result in leaflet tension, or when regional tissue availability is limited (infective lesions, hypoplastic segment, calcification or chordal tethering), a first step should be to attempt mobilizing the available free margin by dividing restrictive chordae to maximize the amount of leaflet surface available for apposition. The next step is to evaluate whether the regional tissue deficit may be corrected by detaching and advancing the adjacent voluminous leaflet segment(s) toward the tissue deficit to recreate enough leaflet surface for deep coaptation with the opposite leaflet without tension (Figures 7,8).
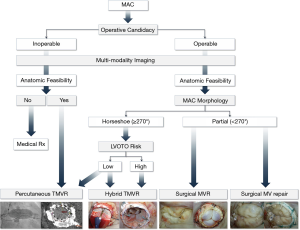
Calcification: the Mount Sinai MAC Algorithm
A common lesion seen with advanced Barlow’s deformity and further precipitated by age and systemic disease, calcification may be limited to the mitral annulus (MAC), or extend to encroach the leaflet, chordae, papillary muscle tips and basal ventricular myocardium. Limited calcification may still allow annular suture placement and a successful repair, with some experts opting to remove the fibrous envelope en-block, with the alternative of a conservative non-resection strategy (leaflet resuspension with placement of artificial chordae) or palliative maneuvers such as edge-to-edge repair. In extensive horseshoe (<270°) or circumferential MAC (≥270°) placement of annular sutures may be hindered, prohibiting the use of annuloplasty devices, while restricting the feasibility of secure deployment of a replacement valve prosthesis. We have developed an algorithm to identify the optimal surgical strategy using advanced imaging and multidisciplinary patient screening to tailor a patient-specific operative approach (7) (Figure 14).
Annuloplasty strategies: rings and bands
Beyond functional reconstruction of the mitral valve leaflets, annuloplasty strategies extend repair durability by restoring the physiologic functional support of the mitral annulus which in turn preserves optimal leaflet coaptation, minimizes stress on the posterior mitral leaflet and posterobasal ventricular myocardium during the cardiac cycle, reinforces the repair suture line and prevents future annular dilatation in myxomatous valve degeneration. While annuloplasty rings and bands can both achieve remodeling of the mitral annulus shape and function, with varying degrees of reduction in the septolateral (SL) and intertrigonal (IT) dimensions, we have increasingly adopted a more conservative strategy with an almost exclusive use of flexible bands in degenerative mitral valve prolapse. The use of a complete semi-flexible ring is reserved for cases where a more pronounced remodeling is desired by fixing the annulus in the systolic position, maintaining the SL/IT ratio of 3:4 throughout the cardiac cycle.
SAM
It is mandatory to identify risk factors for SAM in the preoperative TTE/TEE (i.e., small aorto-mitral angle, basal septal hypertrophy or reduced distance between the point coaptation and basal septum from any etiology). If present, more leaflet resection and/or chordal displacement and an oversized band annuloplasty is recommended (8,9).
Final assessment of repair
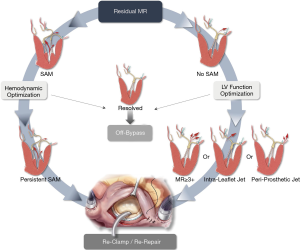
Regardless of the preferred operative approach, long term durability of surgical mitral repair and the associated incremental event-free survival depends primarily on leaving the operating room with no residual or trace MR. The projected durability of the repair result is thus contingent on tension-free, well-supported leaflets, with a posteriorly displaced apposition line, achieved by sufficient coaptation depth of at ~10 mm and reinforced by a true-sized annuloplasty ring or band which affords an unrestricted inflow area and an unobstructed LV outflow. Intraoperative TEE can help assess the final result and identify any such areas of concern. The decision to revise the repair should be carefully assessed, balancing the competing risks between the patient’s baseline morbidities and the long-term impact of the observed hemodynamic profile (i.e., SAM, residual MR). In our mitral reference center we have adopted a zero-tolerance approach for residual MR and developed a decision-making algorithm to stratify the use of a second bypass run based on our experience in employing a revision strategy to effectively mitigate significant MR in select patients (9) (Figure 15).
Take home points
Complex degenerative mitral valve prolapse can be repaired successfully with a methodical assessment of the mechanisms of dysfunction, followed by a lesion-specific approach tailored to the thoughtful control of the excess height, volume and leaflet support, by blending techniques to address all areas of pathology and restore valve competence with long term durability.
Acknowledgments
Funding: The present work was funded in its entirety by the Department of Cardiovascular Surgery at The Icahn School of Medicine at Mount Sinai, NY, USA.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Filip Casselman and Johan van der Merwe) for the series “Aortic and Mitral Valve Innovative Surgery” published in Journal of Visualized Surgery. This article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.com/article/view/10.21037/jovs-2019-12/coif). The series “Aortic and Mitral Valve Innovative Surgery” was commissioned by the editorial office without any funding or sponsorship. DHA serves as the National Co-PI for the following US Pivotal Trials: Medtronic CoreValve US Pivotal Trial, NeoChord System US Pivotal Trial, Medtronic Apollo US Pivotal Trial, Triluminate II US Pivotal Trial; and his institution, the Icahn School of Medicine at Mount Sinai, receives royalty payments from Edwards Lifesciences and Medtronic for intellectual property related to Dr. Adams’ involvement in the development of valve repair rings. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the Helsinki Declaration (as revised in 2013). The manuscript is waived from patient informed consent according to the ethics committee or institutional review board.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Nkomo VT, Gardin JM, Skelton TN, et al. Burden of valvular heart diseases: a population-based study. Lancet 2006;368:1005-11. [Crossref] [PubMed]
- Adams DH, Anyanwu AC. Seeking a Higher Standard for Degenerative Mitral Valve Repair: Begin with Etiology. J Thorac Cardiovasc Surg 2008;136:551-6. [Crossref] [PubMed]
- Castillo JG, Anyanwu AC, Fuster V, et al. A near 100% repair rate for mitral valve prolapse is achievable in a reference center: Implications for future guidelines. J Thorac Cardiovasc Surg 2012;144:308-12. [Crossref] [PubMed]
- Castillo JG, Anyanwu AC, El-Eshmawi A, et al. All anterior and bileaflet mitral valve prolapses are repairable in the modern era of reconstructive surgery. Eur J Cardiothorac Surg 2014;45:139-45. [Crossref] [PubMed]
- Carpentier A, Adams DH, Filsoufi F. Carpentier’s Reconstructive Valve Surgery: From Valve Analysis to Valve Reconstruction. 1st ed. Philadelphia: Saunders/Elsevier, 2010.
- Doherty JU, Kort S, Mehran R, et al. ACC/AATS/AHA/ASE/ASNC/HRS/SCAI/SCCT/SCMR/STS 2017 Appropriate Use Criteria for Multimodality Imaging in Valvular Heart Disease: A Report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery. J Am Coll Cardiol 2017;70:1647-72. [Crossref] [PubMed]
- El-Eshmawi A, Alexis SL, Sengupta A, et al. Surgical management of mitral annular calcification. Curr Opin Cardiol 2020;35:107-15. [Crossref] [PubMed]
- Varghese R, Anyanwu AC, Itagaki S, et al. Management of systolic anterior motion after mitral valve repair: An algorithm. J Thorac Cardiovasc Surg 2012;143:S2-7. [Crossref] [PubMed]
- El-Eshmawi A, Anyanwu A, Boateng P, et al. Second Cross-Clamp to Perfect Degenerative Mitral Valve Repair - Decision Making Algorithm, Safety and Outcomes. J Thorac Cardiovasc Surg 2019;S0022-5223(19)32196-8.
Cite this article as: Rimsukcharoenchai C, Pandis D, El-Eshmawi A, Anyanwu AC, Adams DH. Complex mitral valve regurgitation: surgical evaluation, approach and repair techniques. J Vis Surg 2021;7:15.