
Firefly robotic lung volume reduction surgery: case report
Introduction
Nowadays, video-assisted thoracoscopic surgery (VATS) is the golden standard in lung volume reduction surgery (LVRS), already introduced in 1996 (1). Minimal-invasive techniques offer a clear benefit over thoracotomy by lowering postoperative morbidity (2). Robotic-assisted thoracoscopic surgery (RATS) has been introduced worldwide during the last decade, especially in lung cancer surgery. Recent studies show at least equal safety and feasibility comparing RATS with VATS (3-6). Besides patient selection, identifying the target zone during LVRS is one of the key issues for treatment success. Main part of this process is the thoracic computed tomography (CT), where the surgeon is able to identify emphysema morphology and therefore potential target zones for resection (7,8). Usually, lung perfusion scintigraphy adds value by showing areas with a high perfusion mismatch (9,10). The so called “densitometry” further improves identification of target zones by adding different colors to the CT in relation to areas of low attenuation (11,12).
All these methods are performed pre-operatively and the surgeon needs to transfer the radiological image to the in-situ lung by assessing the macroscopic appearance during thoracoscopy for their correlation to the radiological images and translating this into the resection area.
Besides its potential benefits regarding minimal-invasiveness, the Da Vinci® Xi surgical system provides an integrated fluorescence capability. While intravenously injecting dye, the near-infrared technology called Firefly® is expected to show the perfusion mismatch analogous to emphysema heterogeneity. This might help the surgeon to extend and refine the radiological picture. So far, there is scarce evidence about this topic.
Case presentation
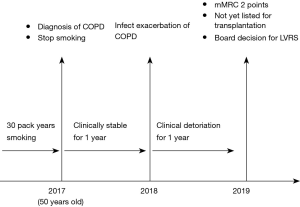
A 51-year-old female patient was referred for LVRS after treatment allocation at the multidisciplinary emphysema treatment board.
The patient was oxygen-dependent, severely impaired in her daily activities and completely incapable of working. The Modified Medical Research Council Dyspnea Scale (mMRC) showed two points. Because of age younger than 55 years, tolerably preserved performance status and osteoporosis as relative contraindication the patient was not yet listed for transplantation. Figure 1 shows the disease-related timeline.
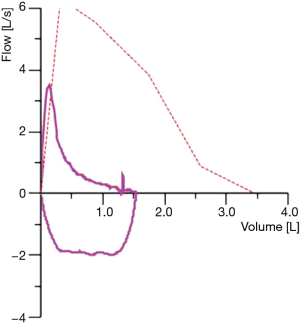
Her pulmonary function test was as follows: forced expiration volume in 1 second (FEV1) 0.8 liters (32% predicted), diffusion capacity 20% and residual volume to total lung capacity ratio (RV/TLC) 59% (Figure 2). She showed a mild post capillary pulmonary hypertension due to a left ventricular diastolic dysfunction by unknown origin. Median pulmonary artery pressure was 25 mmHg, pulmonary artery wedge pressure 16 mmHg.
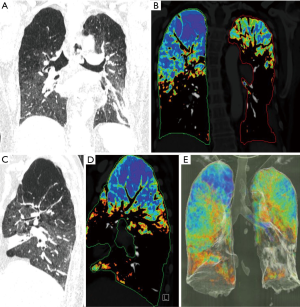
The computer tomography and the perfusion scan showed a markedly heterogeneous, upper lobe predominant emphysema unilaterally on the right (Figure 3).
The patient was scheduled for right-sided, thoracoscopic, robotic-assisted, unilateral LVRS using the Da Vinci® Xi system (Intuitive Surgical Inc., Sunnyvale, CA, USA). During surgery, the integrated fluorescence capability with near-infrared technology (Firefly®) was used. A total amount of 50 mg indocyanine green (Verdye®, Diagnostic Green GmbH, Germany), diluted in 10 mL NaCl, was injected. While injecting the dye via the central line, the less perfused parts of the lung demarcated and helped to identify the most emphysematous and destroyed parts. An upper-lobe hockey-stick like lung volume resection with stapling devices was performed. The ports were closed after putting one chest tube. No epidural anesthesia was performed, the patients was as soon as possible on non-opioid oral analgesics. No adverse or unanticipated events related to the indocyanine green were detected.
The patient had an uneventful postoperative course. The chest tube was removed on the 6th and the patient dismissed on the 7th postoperative day.
The video shows the patients X-rays and thoracic CT, followed by intraoperative views. Identifying the target area by using fluorescence imaging and the resection are presented and discussed. The patient’s written informed consent was obtained. All procedures performed in this study were in accordance with the Helsinki Declaration (as revised in 2013).
Discussion
We presented a case of unilateral robotic assisted thoracoscopic LVRS, supported by using intraoperative indocyanine green (ICG) dye injection and near infrared fluorescence (NIR) by using the Da Vinci camera system being able to switch between real-time and infrared imaging.
LVRS requires precise interpretation of pre-operative imaging as chest CT scans and lung perfusion scintigraphy. The resection target zone in heterogeneous emphysema needs to be separated from areas with preserved tissue. This radiological interpretation has to be translated into the operation room and from the surgeons’ mind onto the patients’ lung (13), which is a particularly challenging process, as resecting too much tissue could be devastating for the patient and resection of too little emphysematous parenchyma might lower the maximal beneficial effect.
Intravenous injection of dye has already been successfully performed in lung surgery, especially in combination with anatomical segmentectomies (14,15). In our case, we used indocyanine green fluorescence as dye, which binds to plasma protein after intravenous injection. Absorbing near infrared light, the dye emits a fluorescence wavelength, which can be seen by the infrared thoracoscopy, integrated in the Da Vinci® system. According to the decreased perfusion in the more destroyed parenchyma parts of a lung with heterogeneous emphysema, this might help to identify the target zone macroscopically. Nevertheless, a precise combination of all methods—imaging and fluoroscopy—is mandatory. Additionally, the surgeon may ask the anesthesiologist to carefully re-ventilate the lung, as seen in the video. Thereby, the more emphysematous parts usually re-ventilate faster than the rest parenchyma with better quality. The more fluorescent parts in our case match quite good with parts that re-expand slower. Nevertheless, it should be integration of both methods supporting the surgeon to the probably ideal resection line. Future developments to imaging integration into robotic systems may further improve the planning and execution of the resection strategy. Although there is so far no evidence for less postoperative pain after RATS compared to VATS, the more gentle aimed robotic arms could be an advantage regarding postoperative pain and therefore potentially less pulmonary complications in this high-risk patient group (16). Additionally, the standardized use of intrathoracic gas during RATS might promote a better approach particularly to the lower lobes by pushing down the diaphragm.
However, the discussion of RATS in thoracic surgery is beyond this case report. The present case demonstrated feasibility and potential benefits for the operative strategy in cases of heterogeneous LVRS. The strength of this article is the current lack of published cases about the concept of fluorescence in LVRS. On the other hand, and adding limitations to the interpretation of this case report, the concept has not been proven yet. Neither larger and more adequate case series have been published nor a qualitative and quantitative comparison of chest CT scans before and after resection, investigation LVRS with and without fluoroscopy, have been performed. At least, the concept seems feasible and warrants more attention.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Jean-Marc Baste) for the series “Robotic Assisted Thoracic Surgery: Advanced Procedures in Lung and Mediastinum: From Post-induction TTT (immunotherapy) to Sleeve Resection, Complex Segmentectomies and Extended Thymectomy for Myasthenia Gravis” published in Journal of Visualized Surgery. This article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.com/article/view/10.21037/jovs-20-73/coif). The series “Robotic Assisted Thoracic Surgery: Advanced Procedures in Lung and Mediastinum: From Post-induction TTT (immunotherapy) to Sleeve Resection, Complex Segmentectomies and Extended Thymectomy for Myasthenia Gravis” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this Case Report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bingisser R, Zollinger A, Hauser M, et al. Bilateral volume reduction surgery for diffuse pulmonary emphysema by video-assisted thoracoscopy. J Thorac Cardiovasc Surg 1996;112:875-82. [Crossref] [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- Yang S, Guo W, Chen X, et al. Early outcomes of robotic versus uniportal video-assisted thoracic surgery for lung cancer: a propensity score-matched study. Eur J Cardiothorac Surg 2018;53:348-52. [Crossref] [PubMed]
- Mahieu J, Rinieri P, Bubenheim M, et al. Robot-Assisted Thoracoscopic Surgery versus Video-Assisted Thoracoscopic Surgery for Lung Lobectomy: Can a Robotic Approach Improve Short-Term Outcomes and Operative Safety? Thorac Cardiovasc Surg 2016;64:354-62. [PubMed]
- Hu X, Wang M. Efficacy and Safety of Robot-assisted Thoracic Surgery (RATS) Compare with Video-assisted Thoracoscopic Surgery (VATS) for Lung Lobectomy in Patients with Non-small Cell Lung Cancer. Comb Chem High Throughput Screen 2019;22:169-78. [Crossref] [PubMed]
- Guo F, Ma D, Li S. Compare the prognosis of Da Vinci robot-assisted thoracic surgery (RATS) with video-assisted thoracic surgery (VATS) for non-small cell lung cancer: A Meta-analysis. Medicine (Baltimore) 2019;98:e17089. [Crossref] [PubMed]
- Weder W, Thurnheer R, Stammberger U, et al. Radiologic emphysema morphology is associated with outcome after surgical lung volume reduction. Ann Thorac Surg 1997;64:313-9; discussion 319-20. [Crossref] [PubMed]
- Russi EW, Bloch KE, et al. Functional and morphological heterogeneity of emphysema and its implication for selection of patients for lung volume reduction surgery. Eur Respir J 1999;14:230-6. [Crossref] [PubMed]
- Thurnheer R, Engel H, Weder W, et al. Role of lung perfusion scintigraphy in relation to chest computed tomography and pulmonary function in the evaluation of candidates for lung volume reduction surgery. Am J Respir Crit Care Med 1999;159:301-10. [Crossref] [PubMed]
- Chandra D, Lipson DA, Hoffman EA, et al. Perfusion scintigraphy and patient selection for lung volume reduction surgery. Am J Respir Crit Care Med 2010;182:937-46. [Crossref] [PubMed]
- Stolk J, Versteegh MI, Montenij LJ, et al. Densitometry for assessment of effect of lung volume reduction surgery for emphysema [published correction appears in Eur Respir J. 2007;30:401.
- Muehlematter UJ, Caviezel C, Martini K, et al. Applicability of color-coded computed tomography images in lung volume reduction surgery planning. J Thorac Dis 2019;11:766-76. [Crossref] [PubMed]
- Caviezel C, Weder W. Lung volume reduction surgery. In: Kaiser L, Jamieson GG, Thompson SK. editor. Operative Thoracic Surgery. 6th edition. CRC Press, 2018.
- Mun M, Okumura S, Nakao M, et al. Indocyanine green fluorescence-navigated thoracoscopic anatomical segmentectomy. J Vis Surg 2017;3:80. [Crossref] [PubMed]
- Motono N, Iwai S, Funasaki A, et al. Low-dose indocyanine green fluorescence-navigated segmentectomy: prospective analysis of 20 cases and review of previous reports. J Thorac Dis 2019;11:702-7. [Crossref] [PubMed]
- Kwon ST, Zhao L, Reddy RM, et al. Evaluation of acute and chronic pain outcomes after robotic, video-assisted thoracoscopic surgery, or open anatomic pulmonary resection. J Thorac Cardiovasc Surg 2017;154:652-659.e1. [Crossref] [PubMed]
Cite this article as: Caviezel C, Schneiter D, Lauk O, Opitz I. Firefly robotic lung volume reduction surgery: case report. J Vis Surg 2021;7:19.


