
Adjunct procedures for malperfusion syndrome in complicated acute type B aortic dissection
Introduction
Acute aortic dissection occurs at a rate exceeding that of aortic aneurysm rupture in the United States (1). The mortality of undiagnosed or delayed diagnosis of acute aortic dissection exceeds 50% at 24 hours (2). Aortic dissection has an incidence of about 2.9–3.5 per 100,000 person years (3,4). There are numerous patient specific factors that have been implicated in the development of aortic dissection, including male gender, a family history of connective tissue disorders, hypertension, and structural abnormalities of the aortic wall.
Dissections of the aorta were historically classified using one of two systems, the Debakey classification and the Stanford classification. The Debakey classification consists of type I (dissection originating in the ascending aorta and extending distally to involve the remainder of the aorta), type II (dissection originating/confined to the ascending aorta), type IIIa (dissection originating in the descending and limited to the descending thoracic aorta) and type IIIb (dissection originating in the descending aorta and involves the abdominal aorta). Stanford type A dissections involve the aortic arch proximal to the innominate artery and type B dissections involve only the thoracic and abdominal aorta distal to the left subclavian artery (5,6). A recent Society of Vascular Surgery and Society of Thoracic Surgery consensus document redefined the anatomic classification and reporting of thoracic aortic dissections. This classification system divides the aorta into 11 anatomic zones extending from the arch (zone 0) to the external iliac artery (zone 11). The dissection is defined as type A if the entry tear involves zone 0 of the aortic arch, and type B if it originates from zone 1 to zone 11. Type A dissections are further defined as AD, where subscript D delineates the zone of distal involvement. Type B dissections are further defined as BP,D where subscript P is the extent of proximal involvement and subscript D is the extent of distal involvement (7).
Approximately 60 percent of patients with aortic dissection present with Type A dissections, while 40 percent present with type B dissections (2,8-10). The hallmark of treatment of type B aortic dissection remains expeditious diagnosis, prompt medical treatment as a first step, and immediate recognition of malperfusion syndromes (1). Once a type B aortic dissection has been diagnosed, prompt medical treatment is the mainstay of initial treatment. This consists of normalizing patient hemodynamics with both blood pressure and impulse control. Most authors and societal guidelines advocate for initial impulse control with intravenous beta-blockers followed by blood pressure control with intravenous vasodilators (11). The 30-day and 1-year mortality of acute type B dissections that are treated medically are favorable compared to those treated surgically in the acute setting. However, patients treated medically require consistent surveillance—long term studies of patients with an acute type B aortic aneurysm treated medically have shown that 29% of patients require aortic surgical intervention in the follow-up period and 38% were dead at an average of 4.3 years of follow up (12).
Complicated aortic dissection: definition and overview of treatment
High-risk aortic dissection is defined as the presence of refractory hypertension (greater than three treatment modalities) and persistent abdominal or back pain that lasts for more than 12 hours despite maximal medical therapy. Another category of high risk aortic dissection is those with concerning radiographic features defined as aorta diameter greater than 40 mm, entry size tear greater than 10 mm, and a proximal entry tear (13,14). Complicated dissections are defined as those with evidence of rupture or end-organ malperfusion either radiographically or clinically. End organ malperfusion manifests as lower limb ischemia, visceral malperfusion, renal failure, stroke, and spinal cord ischemia. Malperfusion syndromes typically manifest as either lower limb malperfusion (LLM) or visceral malperfusion, with varying rates and differing treatment paradigms.
Treatment of the proximal entry tear
Since the 1990s percutaneous methods of revascularization have been increasingly adopted with remarkable success. Malperfusion related to dynamic obstruction by the false lumen may be improved just with proximal coverage of the entry tear using a thoracic aortic endograft (TEVAR), but continued malperfusion after entry tear coverage requires branch vessel stenting. Recent large series of entry tear coverage in complicated type B dissection have shown excellent rates of resolution of ischemia with TEVAR alone. The DISSECTION trial investigated the use of the Medtronic Valiant thoracic endograft in acute complicated type B dissections and demonstrated resolution of malperfusion in 46/50 (92%) of patients treated with proximal TEVAR alone, while 4/50 (8%) required additional endovascular procedures to restore perfusion (15).
Composite device designs have also been shown to be successful in treating complicated type B dissection. Although primarily developed and marketed as an adjunct to prevent chronic degeneration, studies of the Cook dissection stent device using the PETTICOAT technique have demonstrated high rates of resolution for malperfusion syndromes. This device utilizes a covered proximal aortic endograft to cover the entry tear and subsequent bare metal stents distally to expand the true lumen. In the STABLE II trial (16), the Cook dissection stent device was used to treat 73 patients presenting with rupture (20/73) and malperfusion (57/73). All the patient in the study were treated with a proximal stent graft, and the dissection specific stent was utilized in 58/73 patients. Freedom from all-cause mortality was 80% at 1 year, and 9/73 patients required secondary endovascular re-interventions at 1 year. The 1-year results suggest that proximal coverage combined with distal extension with a bare metal dissection stent is a promising first line treatment of malperfusion in complicated type B aortic dissection. This is likely due to the ability of the bare stent to decrease the false lumen area and promote false lumen thrombosis improving perfusion. In the STABLE II trial all patients demonstrated some degree of false lumen thrombosis at 12 months of follow-up (16). Some authors have advocated the addition of balloon angioplasty to the PETTICOAT technique. They theorize that the “stent assisted balloon induced intimal disruption and re-lamination in aortic dissection repair or STABILISE” (17-19) will improve outcomes by creating a single channel aorta that is relined with a composite graft system. Faure et al. reported mid-term outcomes on 41 patients treated with the STABILISE technique, they report 1 death at 30 days and no aortic related deaths at 1 year. They report additional stenting of 15 visceral vessels originating from the false lumen. 39/41 patients had a stable or decreasing aortic diameter, they concluded that STABILISE is a safe and reproducible technique for establishing uni-luminal flow, however longer-term studies are required to evaluate the effect of angioplasty on long term aortic remodeling.
LLM syndrome
LLM syndrome is a relatively common complication of acute aortic dissection. It accounts for up to 73% of malperfusion complications associated with type B dissection (20) and it can be the sole malperfusion syndrome in 52% of cases (20). LLM syndrome is more common in the setting of an acute aortic dissection when compared to chronic long-standing dissection. The majority of patients (87%) present with acute unilateral ischemia and approximately half of patients (56%) present with bilateral ischemia (20).
LLM often presents as an acute surgical emergency. The approach to revascularization of LLM consists of either open surgical or endovascular intervention. The type of open surgical approach utilized depends on presenting symptoms and laterality (bilateral vs. unilateral). LLM presenting in a solitary limb with no other associated malperfusion syndromes is most often re-vascularized with a femoral-femoral bypass. If both lower extremities are malperfused with preserved perfusion of the visceral vessels then an axillary bi-femoral bypass may be performed. Open surgical treatment for LLM in the presence of visceral compromise necessitates open septostomy of the aortic dissection flap with perfusion of the lower extremities as needed either with an in-situ bypass graft or an extra-anatomic bypass.
Endovascular intervention is a viable alternative in patients with suitable anatomy. These techniques have been proposed as a potential intervention since the 1990s and often consist of a hybrid approach of TEVAR with or without additional distal revascularization (21). The initial approach consists of identifying the proximal entry tear and utilizing a thoracic endograft to cover the entry tear and prevent the majority of flow into the false lumen. Distal revascularization is then achieved with stenting to restore the true lumen into non-affected vessels (Figure 1). Another approach is to achieve distal endovascular fenestration of the aortic septum to equalize flow in both the true and false lumens and provide distal perfusion (22). In this case endovascular septostomy is initially formed with a guidewire and guiding catheter, once catheter position is identified to be in the false lumen either by angiography or intravascular ultrasound septectomy is performed. The septectomy is performed using a balloon catheter to further dilate the fenestration and mechanically breakdown the septum separating the two lumens creating two flow channels with more equivalent pressure. This procedure or its modifications utilizing commercially available arterial re-entry catheters to gain entry into the false lumen have been reported in various series with high clinical success rates (23,24). Regardless of the method of fenestration utilized, additional aortic or iliac stent grafting may be needed to ensure proper flow to both lower extremities despite adequate endovascular fenestration.
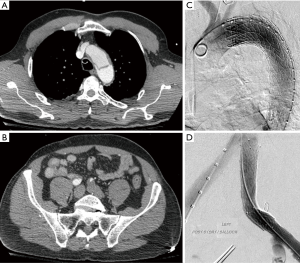
In a systematic review of 29 studies and 138 patients undergoing medical or surgical treatment of LLM syndrome (20), 16% were found to have mild ischemic symptoms and were treated medically, while 37% underwent open surgical treatment and 47% underwent endovascular intervention. 50% of open surgical interventions consisted of an exploratory laparotomy and open fenestration, 46% consisted of extra-anatomic bypass (Axillary bi-femoral/femoral-femoral), and 4% of cases were treated with open thoracic/abdominal aortic reconstruction. In patients treated with endovascular approaches 54% underwent endovascular fenestration, 69% underwent thoracic or abdominal aortic stenting and almost all patients (95%) underwent distal (iliac/femoral) stent grafting. In patients who were treated with open surgical intervention, 27% required re-intervention within 30 days of the index operation and 31% experienced a significant morbidity (acute renal failure, myocardial infarction, paraplegia, paraparesis, chest infection, colectomy, contralateral lower limb ischemia and amputation above the knee). Mortality in patients treated with open surgery was 14% at 30 days of follow up. Comparatively patients treated endovascular interventions required re-intervention in 33% of cases at 30 days, and 46% of patients experienced a significant comorbidity (compartment syndrome with acute renal failure and transient hemodialysis, gastrointestinal ischemia, liver ischemia, isolated acute renal failure, and recurrent implanted stent collapse). The mortality in the endovascular cohort was 8% at thirty days. Endovascular intervention had a higher rate of re-intervention and morbidity but an overall lower mortality compared to open repair which it has largely supplanted as the first-line treatment for LLM syndrome in acute type B aortic dissection (20).
Visceral malperfusion syndrome
Visceral malperfusion occurs in up to 30 percent of patients with acute type A aortic dissection and up to 20 percent in patients with acute type B aortic dissection. The incidence of visceral malperfusion in patients presenting with type B aortic dissection ranges from 13.8% to 25% in most contemporary series. Isolated mesenteric ischemia occurs in about 7% of patients presenting with type B aortic dissection (25).
The diagnosis of visceral malperfusion is more nuanced when compared to LLM due to “soft” physical exam findings such as vague abdominal pain and nausea. Clinically significant laboratory derangements such as elevation of lactate, metabolic acidosis or an elevated creatinine often develop hours after the onset of ischemia potentially obscuring the window of treatment (7). Evidence of end organ damage on CT scan such as bowel compromise also lags significantly behind other clinical findings. Furthermore, the outcome of a delay in diagnosis for visceral malperfusion is associated with a higher mortality compared to a delay in diagnosis of lower extremity malperfusion, as severe visceral insults are often a lethal event (2). Obstruction of the visceral vessels can be a dynamic process secondary to a mobile aortic dissection flap that causes intermittent compression of the true lumen and intermittent visceral ischemia. Alternatively, a static obstruction occurs distal to the area of dissection, with thrombosis of the true lumen distal to the dissection flap secondary to compression by the false lumen at the area of dissection (25,26).
The treatment modalities utilized in the management of the visceral aorta are similar to those employed in managing lower extremity malperfusion. The traditional approach has been open repair with replacement of the visceral aorta with branch vessel bypass, or open surgical fenestration of the dissection flap (25). This approach had an in-hospital mortality rate that approaches 40%, but it did result in the successful treatment of visceral malperfusion in over 90 percent of patients.
Entry tear coverage has recently become the mainstay of treatment. Most malperfusion syndromes including visceral malperfusion respond well to treatment of the entry tear using TEVAR, resulting in decreased perfusion of the false lumen and pressurization of the dissection flap into the visceral arteries. This is often enough to resolve most dynamic obstruction (15) but residual stenosis or occlusion of visceral vessels after proximal entry tear coverage requires subsequent visceral stenting, as seen in the patient treated in Video 1. Stenting of the vessel origins may be technically complicated if the ostium originates from the false lumen or is completely occluded.
Endovascular fenestration is another technique utilized to restore perfusion in complicated type B dissection. A recent study from the University of Michigan detailed the endovascular management of complicated type B aortic dissection in 182 patients spanning the years 1996 to 2018 (27). All these patients were initially treated with endovascular interventions, most with endovascular fenestrations (54%) with or without visceral stenting, with 2.7% requiring concomitant TEVAR. Investigators deemed a pressure gradient of 15 mm of hg across an area of dissection in a visceral vessel to be indicative of a significant stenosis and requiring visceral stenting. They reported a 30-day mortality of 7.6%, a 5-year mortality of 28% and a 10-year mortality of 49%, which is much lower when compared to historical mortality rates after open intervention for visceral malperfusion.
Limitations of current treatment
Despite the much improved mortality rate of endovascular management of malperfusion syndromes, both visceral and that of the lower limb, it remains associated with a certain element of morbidity and mortality. Recent long term analysis of TEVAR vs. medical therapy in uncomplicated type B dissection shows benefit of pre-emptive TEVAR in promoting false lumen thrombosis and decreased aneurysmal degeneration (28). When treating malperfusion without addressing the proximal entry tear, this exposes the thoracic and abdominal aorta to the risk of degenerative changes and subsequent rupture (14). It is unknown whether endovascular fenestration without TEVAR has similar effects on aneurysmal degeneration, therefore there continues to be a slight increase in aneurysmal degeneration or rupture requiring re-intervention in the form of TEVAR. However, TEVAR in acute type B aortic dissection is associated with retrograde type A dissection in 1.7% of cases, with up to 33.6% resultant mortality for repair of the type A dissection. Stent graft associated primary tears occur in up to 3.4% of TEVAR in acute type B aortic dissection with an associated mortality of repair approaching 26.1%. Stroke rate in the acute setting approaches 2%, while the risk of spinal cord ischemia approaches 7% in most registries (27).
Conclusions
Treatment of malperfusion syndromes in complicated type B dissections, require expeditious diagnosis, prompt medical therapy, and often immediate surgical intervention. Endovascular interventions have largely replaced open surgical intervention for complicated type B dissections, especially in visceral malperfusion. There remains a strong role for extra-anatomic open bypass in cases of LLM. They are less morbid and complex procedures that provide a much improved mortality benefit to patients presenting with acute type B dissection.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Ibrahim Sultan and George Arnaoutakis) for the series “Advancement in the Surgical Treatment of Aortic Dissection” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.com/article/view/10.21037/jovs-20-81/coif). The series “Advancement in the Surgical Treatment of Aortic Dissection” was commissioned by the editorial office without any funding or sponsorship. EDA reports personal fees from Boston Scientific, personal fees from Gore Medical, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the Helsinki Declaration (as revised in 2013). The manuscript is waived from patient informed consent according to the ethics committee or institutional review board.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cambria RP. Surgical treatment of complicated distal aortic dissection. Semin Vasc Surg 2002;15:97-107. [Crossref] [PubMed]
- Khan IA, Nair CK. Clinical, diagnostic, and management perspectives of aortic dissection. Chest 2002;122:311-28. [Crossref] [PubMed]
- Clouse WD, Hallett JW Jr, Schaff HV, et al. Acute aortic dissection: population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clin Proc 2004;79:176-80. [Crossref] [PubMed]
- Mészáros I, Mórocz J, Szlávi J, et al. Epidemiology and clinicopathology of aortic dissection. Chest 2000;117:1271-8. [Crossref] [PubMed]
- Daily PO, Trueblood HW, Stinson EB, et al. Management of acute aortic dissections. Ann Thorac Surg 1970;10:237-47. [Crossref] [PubMed]
- Debakey ME, Henly WS, Cooley DA, et al. Surgical Management of Dissecting Aneurysms Of The Aorta. J Thorac Cardiovasc Surg 1965;49:130-49. [Crossref] [PubMed]
- Lombardi JV, Hughes GC, Appoo JJ, et al. Society for Vascular Surgery (SVS) and Society of Thoracic Surgeons (STS) reporting standards for type B aortic dissections. J Vasc Surg 2020;71:723-47. [Crossref] [PubMed]
- Januzzi JL, Isselbacher EM, Fattori R, et al. Characterizing the young patient with aortic dissection: results from the International Registry of Aortic Dissection (IRAD). J Am Coll Cardiol 2004;43:665-9. [Crossref] [PubMed]
- Tsai TT, Evangelista A, Nienaber CA, et al. Long-term survival in patients presenting with type A acute aortic dissection: insights from the International Registry of Acute Aortic Dissection (IRAD). Circulation 2006;114:I350-6. [Crossref] [PubMed]
- Hagan PG, Nienaber CA, Isselbacher EM, et al. The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA 2000;283:897-903. [Crossref] [PubMed]
- Teo EP, Isselbacher EM. Diseases of the Aorta. In: Essential Echocardiography: A Companion to Braunwald’s Heart Disease. Elsevier, 2019.
- Durham CA, Cambria RP, Wang LJ, et al. The natural history of medically managed acute type B aortic dissection. J Vasc Surg 2015;61:1192-8. [Crossref] [PubMed]
- Evangelista A, Salas A, Ribera A, et al. Long-term outcome of aortic dissection with patent false lumen: predictive role of entry tear size and location. Circulation 2012;125:3133-41. [Crossref] [PubMed]
- Durham CA, Cambria RP, Wang LJ, et al. The natural history of medically managed acute type B aortic dissection. J Vasc Surg 2015;61:1192-8. [Crossref] [PubMed]
- Bavaria JE, Brinkman WT, Hughes GC, et al. Outcomes of Thoracic Endovascular Aortic Repair in Acute Type B Aortic Dissection: Results From the Valiant United States Investigational Device Exemption Study. Ann Thorac Surg 2015;100:802-8; discussion 808-9. [Crossref] [PubMed]
- Lombardi JV, Gleason TG, Panneton JM, et al. STABLE II clinical trial on endovascular treatment of acute, complicated type B aortic dissection with a composite device design. J Vasc Surg 2020;71:1077-87.e2. [Crossref] [PubMed]
- Melissano G, Bertoglio L, Rinaldi E, et al. Satisfactory short-term outcomes of the STABILISE technique for type B aortic dissection. J Vasc Surg 2018;68:966-75. [Crossref] [PubMed]
- Hofferberth SC, Nixon IK, Boston RC, et al. Stent-assisted balloon-induced intimal disruption and relamination in aortic dissection repair: the STABILISE concept. J Thorac Cardiovasc Surg 2014;147:1240-5. [Crossref] [PubMed]
- Faure EM, El Batti S, Abou Rjeili M, et al. Mid-term Outcomes of Stent Assisted Balloon Induced Intimal Disruption and Relamination in Aortic Dissection Repair (STABILISE) in Acute Type B Aortic Dissection. Eur J Vasc Endovasc Surg 2018;56:209-15. [Crossref] [PubMed]
- Gargiulo M, Bianchini Massoni C, Gallitto E, et al. Lower limb malperfusion in type B aortic dissection: a systematic review. Ann Cardiothorac Surg 2014;3:351-67. [PubMed]
- Alfson DB, Ham SW, Type B. Aortic Dissections: Current Guidelines for Treatment. Cardiol Clin 2017;35:387-410. [Crossref] [PubMed]
- Norton EL, Williams DM, Kim KM, et al. Management of acute type B aortic dissection with malperfusion via endovascular fenestration/stenting. J Thorac Cardiovasc Surg 2019; [Crossref] [PubMed]
- Midulla M, Renaud A, Martinelli T, et al. Endovascular fenestration in aortic dissection with acute malperfusion syndrome: immediate and late follow-up. J Thorac Cardiovasc Surg 2011;142:66-72. [Crossref] [PubMed]
- Wolfschmidt F, Hassold N, Goltz JP, et al. Aortic Dissection: Accurate Subintimal Flap Fenestration by Using a Reentry Catheter with Fluoroscopic Guidance-Initial Single-Institution Experience. Radiology 2015;276:862-72. [Crossref] [PubMed]
- Kamman AV, Yang B, Kim KM, et al. Visceral Malperfusion in Aortic Dissection: The Michigan Experience. Semin Thorac Cardiovasc Surg 2017;29:173-8. [Crossref] [PubMed]
- Hiratzka LF, Bakris GL, Beckman JA, et al. 2010 ACCF/AHA/AATS/ACR/ASA/SCA/SCAI/SIR/STS/SVM guidelines for the diagnosis and management of patients with thoracic aortic disease: executive summary. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, American Association for Thoracic Surgery, American College of Radiology, American Stroke Association, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of Thoracic Surgeons, and Society for Vascular Medicine. Catheter Cardiovasc Interv 2010;76:E43-86. [Crossref] [PubMed]
- Yang B, Rosati CM, Norton EL, et al. Endovascular Fenestration/Stenting First Followed by Delayed Open Aortic Repair for Acute Type A Aortic Dissection With Malperfusion Syndrome. Circulation 2018;138:2091-103. [Crossref] [PubMed]
- Nienaber CA, Kische S, Rousseau H, et al. Endovascular repair of type B aortic dissection: long-term results of the randomized investigation of stent grafts in aortic dissection trial. Circ Cardiovasc Interv 2013;6:407-16. [Crossref] [PubMed]
Cite this article as: Abdul-Malak OM, Liang NL, Makaroun MS, Avgerinos ED. Adjunct procedures for malperfusion syndrome in complicated acute type B aortic dissection. J Vis Surg 2021;7:44.


