Aortic valve repair—“Pearls and Pitfalls”
Introduction
Current medical evidence shows that aortic valve repair reduces valve-related mortality with a better quality of life compared to prosthetic valve replacement. As a result, the 2017 European Association for Cardio-Thoracic Surgeons (EACTS)/European Society of Cardiology (ESC) guidelines for heart valve disease recommend to spare the valve in patients ‘with pliable, non-calcified’ bicuspid or tricuspid aortic valve insufficiency ‘in whom aortic valve repair may be a feasible alternative to valve replacement’ (class IC indication). They also recommend reimplantation or remodeling with aortic annuloplasty for valve-sparing root replacement, referring to the need of addressing the annulus (1). Indeed a dilated aortic annulus ≥25 mm, if left untreated, is clearly documented as a major risk factor for failure of bicuspid and tricuspid aortic valve repair procedures (2,3). As in the case of mitral valve repair, annuloplasty is now considered as an essential component of aortic valve (AV) repair/sparing procedures, aiming at a sustained long-term outcome by reducing the dilated aortic annulus and protecting the repair by improving the surface of coaptation. This review describes the anatomy behind aortic annuloplasty, the different historical techniques, as well as a standardised approach to aortic valve repair with ring annuloplasty according to each aorta phenotype.
Phenotypes of dystrophic aortic insufficiency (AI)
Dystrophic AI represents the most common aetiology of AI in western countries, accounting for approximately two-thirds of all cases (50% degenerative, 15% congenital) (4) which are good candidates for aortic valve repair. Dystrophic AI is characterized by dilatation of the aortic annulus, sinuses and/or sinotubular junction diameters. When either of these 2 anatomical rings are dilated, the surface of coaptation is reduced, and prolapse of cusp leaflets may be induced. Three phenotypes of AI can be described, depending on whether the sinuses of Valsalva and/or the tubular ascending aorta are dilated: (I) normal root and ascending aorta (all diameters <40–45 mm)—isolated AI; (II) dilatation of the aortic root (sinus of Valsalva ≥45 mm)—root aneurysm; (III) dilatation of the ascending aorta (≥45 mm)—tubular aorta aneurysm (Figure 1) (4).
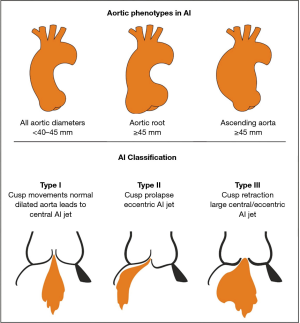
Depending on cusp mobility, there are three types of valve dysfunction: type I characterized by normal cusp motion, associated with root/ascending aorta dilatation (central jet); type II defined as cusp prolapse (eccentric jet); and type III characterized by cusp retraction, associated with poor tissue quality and or quantity (central or eccentric jet). Type I and II are good candidates for repair of bicuspid and tricuspid aortic valves irrespective of the phenotype whereas type III is a more challenging entity for repair and needs to be considered on a case by case basis.
Although patients with dystrophic AI are good candidates for repair, only 1.7% of AI patients have their valve spared. The Society of Thoracic Surgeons’ database analysis showed a slight improvement with 14% of patients who underwent aortic root surgery receiving a valve sparing procedure (20% of low risk and 6% of high risk patients), but still leaving 80% of root procedures for AI and/or root aneurysm as composite valve and graft replacement (Bentall procedure) (5,6). Standardisation of aortic valve repair techniques which address the dilated diameters (sub and supra valvular annuloplasty) for each phenotype as well as cusp resuspension will be key for the dissemination of repair and long-term patient outcomes (Figure 2).

Anatomical landmarks for aortic annuloplasty
The aortic valve is a dynamic complex with a systolic expansibility of the aortic root (6.2% and 5.7% at the aortic annulus and STJ levels respectively) allowing stress-free opening and closure of the cusps (7,8).
Large echocardiographic studies have documented that the sinotubular junction (STJ) [mean 27.2 mm (range, 24.7–29.5)] is larger than the aortic annulus [mean 22.3 mm (range, 20.5–24.5)] with a STJ/aortic annular base ratio of 1.2 (8,9). Therefore, an aortic annulus diameter larger than 25 mm and a STJ diameter larger than 30 mm are deemed as functionally dilated. Indeed finite element analysis has shown that the reduction of STJ induces a symmetrical prolapse by lowering the effective height (eH) of the cusp while dilation of aortic annulus diameter reduces mostly the coaptation height (cH) (10).
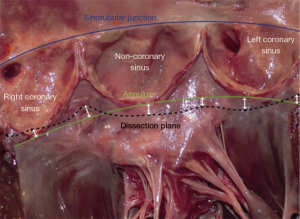
The aortic annulus is a consensus terminology to define the inflow of the aortic root as the plane passing through the nadir of the aortic cusps that can be measured either on echo long axis view or by direct intubation intra operatively (11-16). External dissection of the annulus may be achieved down to the subvalvular level below the nadir of the left and the non-coronary cusps (basal ring), and in 80% of cases below or within 3 mm of the nadir of the right cusp (11,17-21). Of importance to the surgical technique of dissecting down to the subvalvular level, it is difficult to fully reach down to the subvalvular plane in the region below the right-non commissure corresponding to the insertion of the membranous septum, right atrium wall, infundibulum and septal leaflet of the tricuspid valve. Therefore by dissecting down to this deepest plane, the external annuloplasty ring or the proximal suture line of the reimplantation tube graft would fully match the subvalvular plane below at least the left and non-coronary cusps, and remain below or within 3mm of the nadir of the right coronary cusp 80% of the time (Figure 3). The muscular part of the annulus is its thickest portion (with a mean thickness of 2.5 mm). Therefore, an external annuloplasty or proximal suture of a reimplantation would produce a reduction in the annulus of at least 5 mm smaller than the size of the ring or graft (12,22).
Repairing the root—valve-sparing aortic root replacement
Replacement of the aortic root with preservation of the aortic valve has followed predominantly 2 different operations. The remodeling technique uses the tube graft to create 3 scallops or neo-sinuses to replace those of the native aorta. This technique allows root expansion during systole because the interleaflet triangles are not restricted by being placed inside the tube graft (23). The reimplantation technique requires the aortic valve to be placed within a tube graft (24). In both techniques the sinotubular junction is reduced by bringing the commissure to the diameter of the tube. Whereas the remodeling technique preserves the dynamic anatomy of the aortic valve (25), it does not on its own address the annulus. One of the risk factors for recurrent AI and re-operation after the remodeling technique is a dilated annulus (>25 mm) which has been left untreated. This is the case for both bicuspid and tricuspid valves (2,3,26,27). Modification of the remodeling technique to add an external expansile annuloplasty ring has led to significant improvements. In patients with a dilated aortic annulus, the external ring restores the normal annulus diameter and prevents future dilatation (26-31). On the contrary the reimplantation technique does provide an annuloplasty with the proximal suture line. However this technique has inferior haemodynamics to the remodeling technique as there is loss of vortical flow, risk of cusp impaction on the tube graft and rapid valve closure (25). The original reimplantation technique (David I) has also been improved to incorporate a spherical bulb-shaped graft. This has been shown to improve cusp motion and vortical flow in the new root (32,33).
Apart from providing better valve dynamics, the choice of using remodeling + ring relates to both the standardization and reproducibility of valve repair. Whereas in the reimplantation technique the surgeon has to make a judgment on how high to place the commissures and in what circumferential orientation inside the graft, the commissures will follow the graft in the remodeling technique and will therefore be placed at the same level symmetrically. Furthermore, whereas the annuloplasty is the first step carried out in the reimplantation technique through the proximal suture line, it is the last step of the technique in remodeling + ring. Therefore, cusp effective height (see below) is measured in an untouched (often large) annulus, making accurate measurement easier (34-36). Finally, the fallback option in case of valve repair failure, contrary to the reimplantation proximal suture line, the external ring can simply be cut and removed, allowing large prosthetic valve implantation (Figure 4, Table 1).
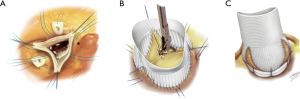
Table 1
| Aortic annulus diameter (Hegar dilator) | ||||
|---|---|---|---|---|
| 25–27 mm | 28–30 mm | 31–35 mm | ≥36 mm | |
| Valsalva graft (mm) | 26 | 28 | 30 | 32 |
| Extra aortic ring (mm) | 25 | 27 | 29 | 31 |
There have been improvements in the long-term outcomes after valve-sparing root procedures. As well as addressing the annulus, this has been in part due to development of surgical techniques to address and repair the aortic valve cusps. Thus, aortic root repair no longer needs to be restricted to patients with insignificant AI. Even the severest forms of AI can be repaired due to advances in cusp management. The systematic measurement of cusp effective height (eH) has allowed the surgeon to assess for cusp prolapse, which may be pre-existing or induced as a result of the valve-sparing root procedure (2,37). The durability of valve-sparing root procedures has been significantly improved by ensuring an intra-operative eH of at least 9mm, and good alignment of cusp free margin length (2).
Isolated aortic valve repair
Isolated dystrophic AI is described when the sinuses of Valsalva and the ascending aorta are both ≤40–45 mm. Despite the aortic diameters being normal, isolated AI is still associated with an enlarged annulus ≥25 mm and/or STJ ≥30 mm in the majority of cases. This signifies the importance of addressing the annulus in all phenotypes of AI.
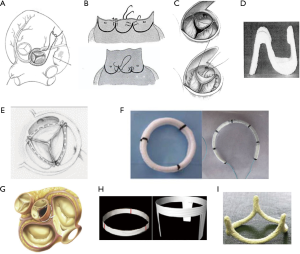
A number of different techniques of addressing the aortic annulus have been performed over the past 60 years (38). The first attempt was in 1958 when “aortic circumclusion” was performed to treat isolated AI by Taylor et al. Silk sutures were placed externally as a circumferential annuloplasty running underneath the coronary arteries on a beating heart (39) (Figure 5). In 1966, Cabrol performed the first internal annuloplasty using sub- and supra-commissural plication sutures of the interleaflet triangle (41,42). This was an attempt at dealing with both the annulus and the STJ. However, high rates of recurrent AI have been reported with redilatation of the aortic annulus for both tricuspid and bicuspid valve (33,43). This technique has therefore fallen out of favour in recent years. Carpentier and subsequently Haydar used a continuous internal suture along the cusp insertion line. Lansac et al. developed double sub- and supravalvular annuloplasty techniques using 2 external rings placed at the annulus and STJ for isolated AV repair in 2003. For the subvalvular ring, the annuloplasty is performed with an open ring passed below the coronaries. This increases the surface of coaptation to protect the repair. Furthermore, a supravalvular annuloplasty is also performed at the level of the STJ in order to restore the STJ/annulus ratio of 1.2 (44-47). In 2009, Schäfers et al. described circumferential suture annuloplasty using polytetrafluoroethylene Gore-Tex 0 suture (3,48). Following Carlos Duran’s description of an internal aortic ring in 1993, Rankin introduced in 2011 a rigid internal ring HAART (Hemispherical Aortic Annuloplasty Ring Technology) (49,50). The majority of these annuloplasty techniques have published in small patient numbers or for short to mid-term follow-up.
External ring aortic annuloplasty: a standardised approach to aortic valve repair
We have developed a standardised approach to AV repair which aims to resuspend the valve and to restore the ratio between STJ/annulus of 1.2. The procedure used is dependent on the phenotype of the aorta (Figure 1); in dilated aortic roots we perform a valve-sparing root replacement (remodeling) with subvalvular annuloplasty; in dilated ascending aorta we perform tubular aorta replacement with subvalvular annuloplasty; and in isolated AI we perform double sub- and supra-valvular annuloplasty. In each of these procedures, both the STJ and the annulus are addressed when the annulus is dilated ≥25 mm; in valve-sparing root procedures, the graft automatically provides a supravalvular STJ annuloplasty by bringing the commissures to the diameter of the tube; the same is true for tubular aorta replacement. In isolated AI, a separate expansible annuloplasty at the supravalvular STJ level in addition to a subvalvular annuloplasty at the annular level using a standardised sizing system would provide both a reduction in respective diameters as well as maintaining the geometric ratio of STJ/annulus and systolic expansibility (Table 2).
Table 2
| Aortic annulus diameter (Hegar dilator) | ||||
|---|---|---|---|---|
| 25–27 mm | 28–30 mm | 31–35 mm | ≥36 mm | |
| Subvalvular aortic ring (mm) | 25 | 27 | 29 | 31 |
| STJ extra aortic ring (mm) | 25 | 27 | 29 | 31 |
STJ, sinotubular junction; AI, aortic insufficiency.
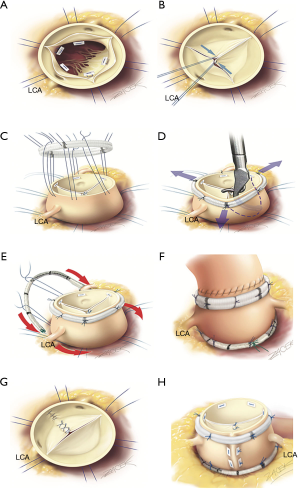
All 3 procedures follow the same steps by performing (I) alignment of cusp free margin; then (II) supravalvular STJ annuloplasty (remodelling for root phenotype, tubular replacement for ascending aorta phentype, STJ ring for isolated AI); followed by (III) cusp effective height assessment; and finally (IV) external ring subvalvular annuloplasty (if the annulus is ≥25 mm) (Video 1).
Since 2003, we have operated 482 patients using this standardized approach with 92% freedom for reoperation at 8 years similar for bicuspid and tricuspid valves according to each phenotype of the proximal aorta. Furthermore, since 2007 we have used systematic effective height assessment and expansible calibrated annuloplasty ring (Extra-Aortic; CORONEO, Inc, Montreal, QC, Canada) with the remodeling process, which has improved freedom from AI grade ≥3 (100%), re-operation (99.1%) and major adverse valve-related events (96.3%) at 7 years follow-up with similar results for bicuspid and tricuspid valve repair (51). Systolo-diastolic expansibility of the annulus was preserved following the annuloplasty (5.1%±9.5%) (52).
The CAVIAAR trial evaluated the safety of valve-sparing root surgery (VSRR) using the remodeling technique with the expansible subvalvular annuloplasty ring, and compared its outcomes with that of the mechanical Bentall procedure. It showed similar 30-day mortality in the 2 treatment groups, with a trend towards more major adverse events in the Bentall group (OR 2.52, P=0.09) (53). At 4 years, crude and propensity matched analyses confirmed that freedom from valve-related death and freedom from hemorrhagic events are significantly higher after valve repair than replacement; respectively 99% vs. 94% (P<0.001) and 89% vs. 78% (P=0.02). Furthermore, freedom from valve related reoperation was similar in the 2 groups (P=0.22).
The importance of STJ stabilization on long-term durability of isolated AI repair has been demonstrated by comparing single ring annuloplasty (subvalvular ring) to double ring annuloplasty (subvalvular and supra-valvular ring) for treatment of isolated AI repair. Double annuloplasty was associated with 100% freedom from recurrence of AI ≥ Grade 3 compared to 67% in the single annuloplasty group at 6 years (P=0.008). Moreover, use of double annuloplasty was correlated with 97% freedom from AV-related reintervention compared to 73% in the single annuloplasty group at 6 years (P=0.02). This technique showed results comparable to those of the valve-sparing procedures at 7 years (52). Long-term survival after AV repair is excellent and similar to sex- and age-matched populations (54).
Bicuspid aortic valve (BAV) repair
BAV can exist in a variety of configurations. BAV repairs in general tend to be more reproducible with the repair outcomes more predictable when compared to tricuspid aortic valves. This is because in the vast majority of cases, the fused cusp has a significant prolapse which requires plication, and the non-fused cusp can be used as a reference point with regards to free margin length.
In BAV repair, the commissural angle between the 2 true commissures is very important to the repair strategy. The commissural angle in a BAV can range anywhere between 120° (very asymmetrical valve, as in the case of a minor form of BAV where the raphe is often very short corresponding to a “tricuspid like configuration”) up to 180° (the very symmetric and rare true type 0 BAV). Often it lies somewhere in the middle. We always aim for a repair strategy which produces a fully symmetrical 180° valve in all cases, with the end result being a symmetrical valve with 2 cusps of equal free margin length, thus mimicking the most stable form of a normal functioning native BAV (the so called “true type 0”). The only exceptions to this rule are those valves where the commissural angle truly lies close to 120° with “tricuspid like configuration”.
In order to create a symmetrical 180° valve, the inter-commissural distance must be adjusted. In most BAVs, the fused sinus is larger than the non-fused sinus. We therefore plicate the fused sinus thereby equalising the inter-commissural distance. We also place the 2 commissures at exactly 180° to each on the STJ ring. These 2 manoeuvres help to create a symmetrical valve configuration.
What is a good repair result, and when to reclamp?
Immediately after removing the cross-clamp, we perform trans-oesophageal echocardiography to assess for AI. This period of time between removing the cross-clamp and discontinuing cardiopulmonary bypass is when the repaired AV is under the most stress. This is because non-pulsatile flow from the arterial cannula is continuously pushing back on the valve. We find that if there is no residual AI at this stage, the results of the repair will be satisfactory once cardiopulmonary bypass has been discontinued.
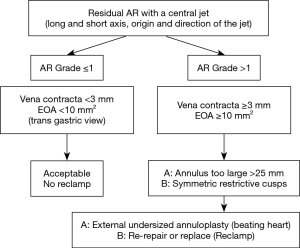
We will only accept no residual AI or trace AI with a central jet. Any eccentric jet, even if trivial, is not accepted as long-term results will be poor. Using 3D echo, we will be satisfied with an effective height eH ≥9 mm, coaptation height (cH) ≥4 mm, an aortic annulus <25 mm, and mean transaortic pressure gradient <10 mmHg (52).
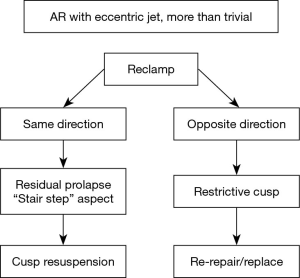
The presence of more than trivial AI after repair, or an eccentric jet, defines a suboptimal result. In this case, the AI jet direction is crucial for subsequent surgical management.
Residual AI with an eccentric jet (Figure 6, Table 2)
Most instances of residual AI (more than trace) with an eccentric jet are an indication to reclamp for a second AV repair, or valve replacement (Figure 7). Eccentric AI may be due to residual cusp prolapse (typically with a “stair step” aspect), which may be treated by repeat cusp resuspension. On the other hand, eccentric AI might be due to cusp restriction, to be treated by the release of some resuspension stitches if possible, or by AV replacement.
AI with a central jet (Figure 8)
Residual central AI is acceptable if it’s not more than trivial, meaning only a limited extension of the color Doppler jet in the LV outflow tract, far from the free edge of the anterior mitral leaflet. Otherwise, the possible causes of significant (grade ≥1/4) residual central AI may be due to an insufficient aortic annuloplasty (annulus ≥25 mm of diameter in systole), or symmetrical over-restriction of the aortic cusps. In the case of insufficient annuloplasty, this can be treated by further undersizing the ring on the beating heart. In the case of symmetrical over-restricted cusps, this can be treated by releasing some of the resuspension stitches if possible, or by AV replacement.
Conclusions
We now have the evidence to show that AV repair is safe, reduces valve-related mortality compared to prosthetic valve replacement, produces better quality of life and provides similar life expectancy as that of the general population. The dissemination and uptake of these techniques very much depends on their standardisation and reproducibility, as well as uniform clinical reporting to evaluate long-term patient outcomes. As part of the standardised technique to treat AI, a calibrated annuloplasty at both sub- and supravalvular levels helps to restore the STJ/annulus ratio, and can be performed according to the different aortic phenotypes such as dilated root, dilated ascending aorta, and isolated AI.
To further analyse the long-term benefits of aortic valve repair, we must continue to search for high quality real-world data from as many institutions as possible. This is the vision of the AVIATOR registry (www.heartvalvesociety.org/AVIATOR).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Filip Casselman and Johan van der Merwe) for the series “Aortic and Mitral Valve Innovative Surgery” published in Journal of Visualized Surgery. The article did not undergo external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.com/article/view/10.21037/jovs-2019-10/coif). The series “Aortic and Mitral Valve Innovative Surgery” was commissioned by the editorial office without any funding or sponsorship. PY was supported by SCTS Ethicon Fellowship. EL has consultant agreements with CORONEO, Inc (www.coroneo.com), in connection with the development of an aortic ring bearing the trade name ‘‘Extra-Aortic.’’ Data extracted from the Heart Valve Society AVIATOR database funded by research grants from Adetec Association and Edwards Life Sciences educational grant. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the Helsinki Declaration (as revised in 2013). The manuscript is waived from patient informed consent according to the ethics committee or institutional review board.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Falk V, Baumgartner H, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur J Cardiothorac Surg 2017;52:616-64. [Crossref] [PubMed]
- Schafers HJ, Raddatz A, Schmied W, et al. Reexamining remodeling. J Thorac Cardiovasc Surg 2015;149:S30-6. [Crossref] [PubMed]
- Schneider U, Aicher D, Miura Y, et al. Suture Annuloplasty in Aortic Valve Repair. Ann Thorac Surg 2016;101:783-5. [Crossref] [PubMed]
- Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003;24:1231-43. [Crossref] [PubMed]
- Detaint D, Jondeau G. Dystrophic aortic insufficiency. Rev Prat 2009;59:187-93. [PubMed]
- Stamou SC, Williams ML, Gunn TM, et al. Aortic root surgery in the United States: A report from the Society of Thoracic Surgeons database. J Thorac Cardiovasc Surg 2015;149:116-22 e4.
- Lansac E, Lim HS, Shomura Y, et al. A four-dimensional study of the aortic root dynamics. Eur J Cardiothorac Surg 2002;22:497-503. [Crossref] [PubMed]
- Lansac E, Di Centa I. Dynamic anatomy to aortic annuloplasty: the tale of the ring. In: Yankah C, Weng Y, Hetzer R, editors. Aortic Root Surgery: The biological Solution. Berlin Heigdelberg: Springer-Verlag, 2010: 102-32.
- Bierbach BO, Aicher D, Issa OA, et al. Aortic root and cusp configuration determine aortic valve function. Eur J Cardiothorac Surg 2010;38:400-6. [Crossref] [PubMed]
- Marom G, Haj-Ali R, Rosenfeld M, et al. Aortic root numeric model: annulus diameter prediction of effective height and coaptation in post-aortic valve repair. J Thorac Cardiovasc Surg 2013;145:406-11.e1. [Crossref] [PubMed]
- Sievers HH, Hemmer W, Beyersdorf F, et al. The everyday used nomenclature of the aortic root components: the tower of Babel? Eur J Cardiothorac Surg 2012;41:478-82. [Crossref] [PubMed]
- Anderson RH. Demolishing the tower of babel. Eur J Cardiothorac Surg 2012;41:483-4. [Crossref] [PubMed]
- Frater RW, Anderson RH. How can we logically describe the components of the arterial valves? J Heart Valve Dis 2010;19:438-40. [PubMed]
- Anderson RH, Devine WA, Ho SY, et al. The myth of the aortic annulus: the anatomy of the subaortic outflow tract. Ann Thorac Surg 1991;52:640-6. [Crossref] [PubMed]
- Sutton JP 3rd, Ho SY, Anderson RH. The forgotten interleaflet triangles: a review of the surgical anatomy of the aortic valve. Ann Thorac Surg 1995;59:419-27. [Crossref] [PubMed]
- de Kerchove L, El Khoury G. Anatomy and pathophysiology of the ventriculo-aortic junction: implication in aortic valve repair surgery. Ann Cardiothorac Surg 2013;2:57-64. [PubMed]
- de Kerchove L, Jashari R, Boodhwani M, et al. Surgical anatomy of the aortic root: implication for valve-sparing reimplantation and aortic valve annuloplasty. J Thorac Cardiovasc Surg 2015;149:425-33. [Crossref] [PubMed]
- Khelil N, Sleilaty G, Palladino M, et al. Surgical anatomy of the aortic annulus: landmarks for external annuloplasty in aortic valve repair. Ann Thorac Surg 2015;99:1220-6. [Crossref] [PubMed]
- Lansac E, de Kerchove L. Aortic valve repair techniques: state of the art. Eur J Cardiothorac Surg 2018;53:1101-7. [Crossref] [PubMed]
- Anderson RH, Mori S. Nomenclature of the components of the aortic root. Eur J Cardiothorac Surg 2019;55:1020. [Crossref] [PubMed]
- Lansac E, de Kerchove L. Reply to Anderson and Mori. Eur J Cardiothorac Surg 2019;55:1020-1. [Crossref] [PubMed]
- Roman MJ, Devereux RB, Niles NW, et al. Aortic root dilatation as a cause of isolated, severe aortic regurgitation. Prevalence, clinical and echocardiographic patterns, and relation to left ventricular hypertrophy and function. Ann Intern Med 1987;106:800-7. [Crossref] [PubMed]
- Sarsam MA, Yacoub M. Remodeling of the aortic valve anulus. J Thorac Cardiovasc Surg 1993;105:435-8. [Crossref] [PubMed]
- David TE, Feindel CM. An aortic valve-sparing operation for patients with aortic incompetence and aneurysm of the ascending aorta. J Thorac Cardiovasc Surg 1992;103:617-21; discussion 622. [Crossref] [PubMed]
- Ranga A, Bouchot O, Mongrain R, et al. Computational simulations of the aortic valve validated by imaging data: evaluation of valve-sparing techniques. Interact Cardiovasc Thorac Surg 2006;5:373-8. [Crossref] [PubMed]
- Lansac E, Di Centa I, Varnous S, et al. External aortic annuloplasty ring for valve-sparing procedures. Ann Thorac Surg 2005;79:356-8. [Crossref] [PubMed]
- Lansac E, Di Centa I, Bonnet N, et al. Aortic prosthetic ring annuloplasty: a useful adjunct to a standardized aortic valve-sparing procedure? Eur J Cardiothorac Surg 2006;29:537-44. [Crossref] [PubMed]
- Lansac E, Di Centa I, Raoux F, et al. Aortic annuloplasty: towards a standardized approach of conservative aortic valve surgery. Multimed Man Cardiothorac Surg 2007;2007:mmcts.2006.001958.
- Lansac E, Di Centa I, Raoux F, et al. An expansible aortic ring for a physiological approach to conservative aortic valve surgery. J Thorac Cardiovasc Surg 2009;138:718-24. [Crossref] [PubMed]
- Basmadjian L, Basmadjian AJ, Stevens LM, et al. Early results of extra-aortic annuloplasty ring implantation on aortic annular dimensions. J Thorac Cardiovasc Surg 2016;151:1280-5.e1. [Crossref] [PubMed]
- Lenoir M, Maesen B, Stevens LM, et al. Reimplantation versus remodelling with ring annuloplasty: comparison of mid-term outcomes after valve-sparing aortic root replacement. Eur J Cardiothorac Surg 2018;54:48-54. [Crossref] [PubMed]
- Oechtering TH, Hons CF, Sieren M, et al. Time-resolved 3-dimensional magnetic resonance phase contrast imaging (4D Flow MRI) analysis of hemodynamics in valve-sparing aortic root repair with an anatomically shaped sinus prosthesis. J Thorac Cardiovasc Surg 2016;152:418-27.e1. [Crossref] [PubMed]
- de Kerchove L, Boodhwani M, Glineur D, et al. Valve sparing-root replacement with the reimplantation technique to increase the durability of bicuspid aortic valve repair. J Thorac Cardiovasc Surg 2011;142:1430-8. [Crossref] [PubMed]
- Youssefi P, Di Centa I, Khelil N, et al. Valve sparing root replacement: remodeling root repair with aortic ring annuloplasty. Ann Cardiothorac Surg 2019;8:411-4. [Crossref] [PubMed]
- Youssefi P, Zacek P, Debauchez M, et al. Valve-Sparing Aortic Root Replacement Using the Remodeling Technique With Aortic Annuloplasty: Bicuspid Valves With Repair of Specific Lesion Sets: How I Teach It. Ann Thorac Surg 2019;108:324-33. [Crossref] [PubMed]
- Youssefi P, Zacek P, Debauchez M, et al. Valve-Sparing Aortic Root Replacement Using the Remodeling Technique With Aortic Annuloplasty: Tricuspid Valves With Repair of Specific Lesion Sets: How I Teach It. Ann Thorac Surg 2019;107:1592-9. [Crossref] [PubMed]
- Schafers HJ, Bierbach B, Aicher D. A new approach to the assessment of aortic cusp geometry. J Thorac Cardiovasc Surg 2006;132:436-8. [Crossref] [PubMed]
- Youssefi P, El-Hamamsy I, Lansac E. Rationale for aortic annuloplasty to standardise aortic valve repair. Ann Cardiothorac Surg 2019;8:322-30. [Crossref] [PubMed]
- Taylor WJ, Thrower WB, Black H, et al. The surgical correction of aortic insufficiency by circumclusion. J Thorac Surg 1958;35:192-205 passim. [Crossref] [PubMed]
- Kunihara T. Annular management during aortic valve repair: a systematic review. Gen Thorac Cardiovasc Surg 2016;64:63-71. [Crossref] [PubMed]
- Duran CG. Reconstructive techniques for rheumatic aortic valve disease. J Card Surg 1988;3:23-8. [Crossref] [PubMed]
- Cosgrove DM, Rosenkranz ER, Hendren WG, et al. Valvuloplasty for aortic insufficiency. J Thorac Cardiovasc Surg 1991;102:571-6; discussion 576-7. [Crossref] [PubMed]
- le Polain de Waroux JB, Pouleur AC, Goffinet C, et al. Functional anatomy of aortic regurgitation: accuracy, prediction of surgical repairability, and outcome implications of transesophageal echocardiography. Circulation 2007;116:I264-9. [Crossref] [PubMed]
- Youssefi P, Zacek P, Debauchez M, et al. Isolated Tricuspid Aortic Valve Repair With Double Annuloplasty: How I Teach It. Ann Thorac Surg 2019;108:987-94. [Crossref] [PubMed]
- Youssefi P, Brega C, Shraer N, et al. Isolated Bicuspid Aortic Valve Repair With Double Annuloplasty: How I Teach It. Ann Thorac Surg 2019;108:1596-604. [Crossref] [PubMed]
- Schollhorn J, Rylski B, Beyersdorf F. Aortic valve annuloplasty: new single suture technique. Ann Thorac Surg 2014;97:2211-3. [Crossref] [PubMed]
- Haydar HS, He GW, Hovaguimian H, et al. Valve repair for aortic insufficiency: surgical classification and techniques. Eur J Cardiothorac Surg 1997;11:258-65. [Crossref] [PubMed]
- Schneider U, Hofmann C, Aicher D, et al. Suture Annuloplasty Significantly Improves the Durability of Bicuspid Aortic Valve Repair. Ann Thorac Surg 2017;103:504-10. [Crossref] [PubMed]
- Schomburg JL, Lahti MT, Ruth GR, et al. Internal aortic annuloplasty: a novel technique. J Invest Surg 2011;24:222-6. [Crossref] [PubMed]
- Rankin JS, Conger JL, Tuzun E, et al. In vivo testing of an intra-annular aortic valve annuloplasty ring in a chronic calf model. Eur J Cardiothorac Surg 2012;42:149-54. [Crossref] [PubMed]
- Lansac E, Di Centa I, Sleilaty G, et al. Remodeling root repair with an external aortic ring annuloplasty. J Thorac Cardiovasc Surg 2017;153:1033-42. [Crossref] [PubMed]
- Lansac E, Di Centa I, Sleilaty G, et al. Long-term results of external aortic ring annuloplasty for aortic valve repair. Eur J Cardiothorac Surg 2016;50:350-60. [Crossref] [PubMed]
- Lansac E, Bouchot O, Arnaud Crozat E, et al. Standardized approach to valve repair using an expansible aortic ring versus mechanical Bentall: early outcomes of the CAVIAAR multicentric prospective cohort study. J Thorac Cardiovasc Surg 2015;149:S37-45. [Crossref] [PubMed]
- Zakkar M, Bruno VD, Zacek P, et al. Isolated aortic insufficiency valve repair with external ring annuloplasty: a standardized approach. Eur J Cardiothorac Surg 2020;57:308-16. [PubMed]
Cite this article as: Youssefi P, Lansac E, Zacek P, Berrebi A, Czytrom D, Mankoubi L, Noghin M, Diakov C, Monin JL, Debauchez M. Aortic valve repair—“Pearls and Pitfalls”. J Vis Surg 2020;6:36.