Current laparoscopic technique of magnetic sphincter augmentation device implantation
Introduction
Gastroesophageal reflux disease (GERD) is a commonly encountered medical condition affecting individuals across the globe. Multiple treatment strategies have been devised, including initial attempts of lifestyle modifications followed by medical management with proton pump inhibitors (PPIs) or histamine blockers. For refractory cases, procedural interventions are frequently utilized, specifically gastroesophageal (GE) fundoplication, in order to recreate the lower esophageal sphincter mechanism and prevent ongoing reflux disease. However, this operative procedure is not without risks and certain downsides. From a patient perspective, some worry about elevated levels of flatulence, inability to belch or vomit, the durability, and the reproducibility (1).
As a result, an alternative procedure to undergoing fundoplication was developed utilizing a magnetic sphincter augmentation (MSA) device to control reflux symptoms and augment the native valve. This device consists of a series of rare earth magnets encased in a titanium shell and supported with titanium struts between each bead that is fitted around the GE junction. It has an internal resting pressure that, when the device is closed, prevents reflux of gastric contents into the esophagus. Conversely, it allows the device to open in response to increased intraluminal pressure during esophageal contraction and passage of a food bolus. The LINXTM reflux management system (Torax Medical Inc., Shoreview, MN, USA) was approved for use in the United States by the Food and Drug Administration in 2012 for the treatment of GERD.
Initially, the magnetic sphincter was implanted using a minimal dissection technique that avoided disrupting the native attachments around the esophageal hiatus. This has rapidly given way to a full dissection technique that restores esophageal length and closes the hiatal opening before implanting the device. This article describes the current implantation technique of the MSA device but also discusses patient selection, postoperative care, and current outcome data for placement of MSA devices.
Indications and patient selection
The main indication for the MSA procedure is a diagnosis of GERD with persistent symptoms despite trials of lifestyle modification and medical therapy. Ideal patients have normal esophageal motility, either no or small hiatal hernias (<3 cm), and Los Angeles Grade A or B esophagitis or better. In recent years, use of the MSA has expanded to include patients with larger hiatal hernias, esophageal dysmotility, Barrett’s esophagus, as well as patients with reflux symptoms after sleeve gastrectomy, though the data is limited in these patient populations. The only true contraindications to implanting the device are esophageal cancer and allergies to titanium or other metals (2).
Implantation technique
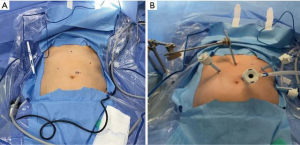
For this procedure, the patient is placed in low lithotomy, allowing the primary surgeon to stand at the caudal aspect between the stirrups with an assistant on the patient’s left. Five incision sites are utilized (Figure 1):
- 11 mm port at the left superolateral aspect of umbilicus for the laparoscope;
- 5 mm subxiphoid port for Nathanson liver retractor;
- 2 separate 5 mm left costal margin ports;
- 5 mm right subcostal port.
From the beginning of the operation, there are multiple important anatomical landmarks of which to be cognizant, specifically surrounding the initial dissection. Both the anterior and posterior branches of the vagus nerve should be identified early and preserved. Additionally, adequate dissection and exposure of the crura bilaterally is essential to (I) facilitate proper evaluation of a hiatal hernia, as this must be fully reduced prior to implantation, (II) assess for adequate intra-abdominal esophageal length (2–3 cm), and (III) place crural approximation sutures to prevent re-herniation and restore a major component of the reflux barrier. Adequate sizing is also prudent for patient safety and procedure efficacy to prevent patient morbidity related to having a device that is either too loose or tight (i.e., additional operations, device erosion, etc.).
To begin this operation, pneumoperitoneum is established. Accessory ports are inserted as above, with placement of the Nathanson liver retractor in the subxiphoid incision for adequate exposure of the GE junction. It is essential to establish appropriate liver retraction at this time for optimal visualization, subsequent success during dissection and evaluation of the GE junction, and procedural efficiency.
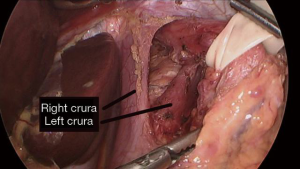
Dissection of the GE junction is then performed utilizing either monopolar cautery or a surgical sealing device in order to isolate the posterior esophageal space (see Video 1 and Figure 2). This is supplemented with atraumatic graspers and Maryland dissectors. The gastrohepatic ligament is incised and divided proximally for exposure of the right crura, with caution to preserve the hepatic branch of the left vagus nerve. This is followed by division of the phrenoesophageal ligament wrapping anteriorly around the esophagus. Great care is taken to identify and preserve both the posterior and anterior vagus nerves, their subsequent branches, as well as the left gastric artery and its branches to avoid injury to these structures. Dissection continues behind the esophagus to create a retroesophageal tunnel. Once this is established, a Penrose drain is placed in that space and wrapped around the esophagus to facilitate manipulation and evaluation of the GE junction.
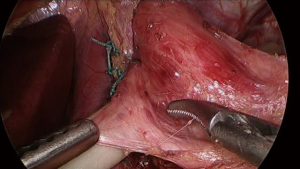
After creation of the retroesophageal tunnel, the presence and degree of a hiatal hernia is assessed. Ideally, at least three centimeters of intraabdominal esophageal length is established, though two centimeters is the absolute minimum recommended if unable to safely obtain more length. Once appropriate intraabdominal length is achieved, the posterior crura are approximated using “0” polyester suture in an interrupted fashion. Subsequently, the posterior vagus nerve is again identified and carefully dissected away from the esophagus utilizing a Maryland dissector as well as an atraumatic grasper for lateral retraction of the tissue. The placement of the Penrose drain is adjusted to exclude and protect the nerve (See Video 2 and Figure 3).
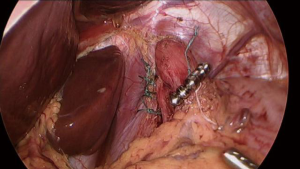
At this time, sizing of the magnetic sphincter device is performed (See Video 3 and Figure 4). Just prior to inserting the sizing instrument, the posterior vagus is once more identified and protected. The sizing instrument is inserted into the right subcostal port and advanced along the anterior aspect of the Penrose into the retroesophageal space. The inner tubing of the sizing device is advanced, and the entire instrument is pulled back to allow the inner tube to wrap around the GE junction and the magnetic end to attach to the distal sizing handle. The inner tubing is carefully tightened so that it rests without pressure along the external aspect of the GE junction, and the corresponding size is noted via the external markings on the sizing device on the proximal aspect of the instrument. Once the same size has been noted on three different measurement attempts and it “pops” open at the same size, we add 2 beads above that measurement to choose the correct size. For example, if the sizing device uncouples at a size 14, then a 16-bead device is chosen. The sizing instrument is removed.
The MSA device is inserted with a grasper and pulled through the retroesophageal space, and the magnetic ends are brought together for attachment on the anterior aspect of the GE junction. The green suture is pulled anterior toward a 12 o’clock position and the white suture is pulled inferior toward a 6 o’clock position to orient the clasp. Once partially closed, the left-hand grasper pinches the clasp together while holding the white suture with the right hand. After this, the left hand holds the green suture, and the right hand pinches the clasp. It should now be closed. At this point, we subsequently perform an intraoperative endoscopy to ensure that the device lies just at the top of the rugal folds when visualized by endoscopy.
As with other laparoscopic procedures, the patient is flattened, and the ports are removed under direct visualization prior to desufflation of the abdomen. The port sites are closed in a standard fashion, and the patient is taken to post-anesthesia care unit (PACU) for initial postoperative monitoring.
Postoperative care
After the procedure, patients are extubated and are discharged home within 4–5 hours after the procedure. As is common with most antireflux operations, pain and nausea control are paramount and serve as the mainstay of postoperative care. Our usual choices for pain control are liquid acetaminophen and liquid ibuprofen supplemented with liquid hydrocodone. Nausea is controlled with ondansetron and metoclopramide. Patients are started on a clear liquid diet immediately postoperatively once appropriately recovered from anesthesia. The morning after surgery they are instructed to breakfast with soft foods such as eggs, with a normal diet beginning at lunch the day after surgery. For the first 6 weeks, they are instructed to eat a small amount of food every hour they are awake to “exercise” or activate the device as many times as possible. The amount of food for these frequent meals should fit in the palm of their hand. They are seen in clinic for follow-up roughly 2 weeks after the procedure to monitor for success and address any postoperative concerns the patient may have.
Discussion
Since its approval in 2012, implantation of MSA has resulted in consistent results with many studies demonstrating the efficacy of this device. Single arm studies have generally reported on short-term outcomes at the 1-year of follow up. These single arm studies show consistent improvement in symptom control as evidenced by significant improvements in GERD-HRQL scores as well as freedom from PPIs ranging from 76–100% (1,6-8). Objective evidence of reflux assessed by pH probe analysis show a range of normalization of pH from 56% to 90%, including the MSA arm of the comparative trials below (1,7-9).
At present, there is only limited long-term (5 years or greater) follow up data. The largest study by Ganz et al. reported, on the long-term outcomes of 100 patients who formed the initial study cohort (10). With an 85% (85/100 patients) 5-year follow up rate, 83% of patients achieved a 50% or greater reduction in GERD-HRQL scores, and median GERD-HRQL scores off PPIs decreased from 27 at baseline to 4 after 5 years post-surgery (P<0.001). Moreover, they obtained a decrease in patients who reported moderate to severe heartburn from 89% to 11.9%, and regurgitation symptoms dropped from 57% to 1.2% (P<0.001 for all comparisons). When analyzing use of PPIs, 75.3% of patients were completely off medication at 5 years, and 9.4% used them only as needed (10).
There is one randomized control trial comparing MSA to double dose PPIs and no randomized trial comparing MSA to laparoscopic antireflux surgery. Not surprisingly, MSA was superior to double dose PPIs particularly in regurgitation dominant patients (11). When compared to Nissen fundoplication, MSA results in similar improvements in GERD-HRQL and similar rates of freedom from PPIs in the studies reporting that outcome, aside from one study that demonstrated higher PPI use in MSA with a P value of 0.02 (7,12,13).
As with any operation, there are risks to consider for implantation of the magnetic sphincter device. The most common side effect reported by patients is dysphagia in the early postoperative period, though many will experience resolution with time. Ayazi et al. demonstrated 15.5% of patients reported continued dysphagia that was still present beyond the third postoperative month, with 30.5% of patients requiring at least 1 dilation (1). They noted an increased risk of such dysphagia in patients without a hiatal hernia, with decreased GI motility, or with dysphagia present preoperatively (1). Comparatively, the 5-year results of the Ganz study demonstrated a 6% rate of “bothersome dysphagia” (10). Other more severe complications associated with this device are migration and erosion (0–0.1%), though they are significantly less commonly observed than dysphagia (7,10,12,14,15).
How the change in implantation technique has impacted these outcomes is unclear, because this change happened clinically rather than through prospective study. There are three papers that have assessed the role of crural closure during MSA implantation. The first compared patients with no hiatal hernia and presumably underwent minimal dissection to implant the device to patients with a hiatal hernia who underwent MSA placement with repair of the hernia. Despite the results being similar, including pH normalization, the authors concluded hiatal hernia closure was necessary (16). The second study found that patients who underwent hiatal hernia repair/crural closure resulted in less recurrent GERD, which was defined by PPI resumption (17). The last study compared different methods of hiatal closure using pH normalization as the primary outcome and, on multivariable regression analysis, shows that full dissection of the hiatus, restoration of intra-abdominal length, and crural closure was more likely to lead to pH normalization than other methods. However, this study identified that full dissection does not always lead to normalization of the post MSA pH testing (18).
One concern possibly related to the change from minimal dissection implantation to full crural dissection and closure could be the change in rates of dysphagia reported in recent MSA papers. In the original 100 patient trial and the 5-year follow up, dysphagia rates were 11% after 1 year and 6% after 5 years compared to a baseline rate of 5% (9). However, in later post approval studies, they rose to 8.7% in one (19) and to 15.5% in another, the latter of which noted a decreased need for dilation after adjustment of their sizing protocol (1). In an attempt to close the crura snugly, surgeons may be creating dysphagia by increasing LES pressure and, when combined with scarring around the device, create a cicatrix that creates a holdup during swallowing (16,17). To counter this, some MSA surgeons are also selecting a device size 3 beads larger than when the sizing device uncouples to mitigate the development of dysphagia. More data is clearly required to understand the balance between GERD control, scarring, and dysphagia.
Conclusions
MSA provides an additional safe and effective strategy for combating symptoms of GERD beyond medications and fundoplication. The current implantation technique consists of common straightforward steps that begin like a fundoplication and end with placement of the device. MSA has provided consistent improvements in GERD symptoms in the majority of patients but not always pH control. Complete hiatal dissection, crural closure, and placement of an MSA device has yielded higher rates of pH normalization, but a rising post-operative dysphagia rate suggests further balancing between the pH control and symptom control may be warranted.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Douglas Z Liou) for the series “Advancement in Treatment for Esophageal Diseases” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.com/article/view/10.21037/jovs.2020.02.06/coif). The series “Advancement in Treatment for Esophageal Diseases” was commissioned by the editorial office without any funding or sponsorship. BEL has had research relationship with Torax Medical Inc., having received a grant for a research study related to the LINX device. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the Helsinki Declaration (as revised in 2013).The manuscript is waived from patient informed consent according to the ethics committee or institutional review board.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ayazi S, Zheng P, Zaidi AH, et al. Magnetic sphincter augmentation and postoperative dysphagia: characterization, clinical risk factors, and management. J Gastrointest Surg 2020;24:39-49. [Crossref] [PubMed]
- Mihura M, Louie BE. Laparoscopic antireflux surgery: magnetic gej augmentation. In: Grams J, Perry KA, Tavakkoli A. editors. The SAGES manual of foregut surgery. Cham: Springer International Publishing, 2019:171-92.
- Ohe KN, Louie BE. Mediastinal dissection with isolation of the GE junction utilizing a Penrose drain. Asvide 2020;7:077. Available online: http://www.asvide.com/watch/33116
- Ohe KN, Louie BE. Crural approximation. Asvide 2020;7:078. Available online: http://www.asvide.com/watch/33117
- Ohe KN, Louie BE. Sizing and placement of magnetic sphincter device. Asvide 2020;7:079. Available online: http://www.asvide.com/watch/33119
- Reynolds JL, Zehetner J, Bildzukewicz N, et al. Magnetic sphincter augmentation with the LINX device for gastroesophageal reflux disease after U.S. Food and Drug Administration approval. Am Surg 2014;80:1034-8. [PubMed]
- Louie BE, Farivar AS, Shultz D, et al. Short-term outcomes using magnetic sphincter augmentation versus Nissen fundoplication for medically resistant gastroesophageal reflux disease. Ann Thorac Surg 2014;98:498-504; discussion 504-5. [Crossref] [PubMed]
- Bonavina L, DeMeester T, Fockens P, et al. Laparoscopic sphincter augmentation device eliminates reflux symptoms and normalizes esophageal acid exposure: one- and 2-year results of a feasibility trial. Ann Surg 2010;252:857-62. [Crossref] [PubMed]
- Ganz RA, Peters JH, Horgan S, et al. Esophageal sphincter device for gastroesophageal reflux disease. N Engl J Med 2013;368:719-27. [Crossref] [PubMed]
- Ganz RA, Edmundowicz SA, Taiganides PA, et al. Long-term Outcomes of Patients Receiving a Magnetic Sphincter Augmentation Device for Gastroesophageal Reflux. Clin Gastroenterol Hepatol 2016;14:671-7. [Crossref] [PubMed]
- Bell R, Lipham J, Louie B, et al. Laparoscopic magnetic sphincter augmentation versus double-dose proton pump inhibitors for management of moderate-to-severe regurgitation in GERD: a randomized controlled trial. Gastrointest Endosc 2019;89:14-22.e1. [Crossref] [PubMed]
- Reynolds JL, Zehetner J, Wu P, et al. Laparoscopic magnetic sphincter augmentation vs laparoscopic Nissen fundoplication: a matched-pair analysis of 100 patients. J Am Coll Surg 2015;221:123-8. [Crossref] [PubMed]
- Warren HF, Reynolds JL, Lipham JC, et al. Multi-institutional outcomes using magnetic sphincter augmentation versus Nissen fundoplication for chronic gastroesophageal reflux disease. Surg Endosc 2016;30:3289-96. [Crossref] [PubMed]
- Saino G, Bonavina L, Lipham JC, et al. Magnetic sphincter augmentation for gastroesophageal reflux at 5 years: final results of a pilot study show long-term acid reduction and symptom improvement. J Laparoendosc Adv Surg Tech A 2015;25:787-92. [Crossref] [PubMed]
- Lipham JC, Taiganides PA, Louie BE, et al. Safety analysis of first 1000 patients treated with magnetic sphincter augmentation for gastroesophageal reflux disease. Dis Esophagus 2015;28:305-11. [Crossref] [PubMed]
- Schwameis K, Nikolic M, Castellano DGM, et al. Crural closure improves outcomes of magnetic sphincter augmentation in GERD patients with hiatal hernia. Sci Rep 2018;8:7319. [Crossref] [PubMed]
- Tatum JM, Alicuben E, Bildzukewicz N, et al. Minimal versus obligatory dissection of the diaphragmatic hiatus during magnetic sphincter augmentation surgery. Surg Endosc 2019;33:782-8. [Crossref] [PubMed]
- Irribarra MM, Blitz S, Wilshire CL, et al. Does treatment of the hiatus influence the outcomes of magnetic sphincter augmentation for chronic GERD? J Gastrointest Surg 2019;23:1104-12. [Crossref] [PubMed]
- Louie BE, Smith CD, Smith CC, et al. Objective evidence of reflux control after magnetic sphincter augmentation: one year results from a post approval study. Ann Surg 2019;270:302-8. [Crossref] [PubMed]
Cite this article as: Ohe KN, Louie BE. Current laparoscopic technique of magnetic sphincter augmentation device implantation. J Vis Surg 2020;6:28.