
Robotic cystgastrostomy and transgastric pancreatic debridement: case report
Introduction
Cystgastrostomy is indicated for the treatment of symptomatic wall off pancreatic necrosis (WOPN) and pancreatic pseudocysts that abut the stomach and do not spontaneously resolve (1). Walled off pancreatic necrosis is the sequelae of necrotizing pancreatitis and is defined as a walled off collection of fluid, necrotic fat, and/or pancreatic tissue that persists 4 weeks beyond the episode of pancreatitis (2). A pancreatic pseudocyst, by contrast, is the sequelae of acute interstitial pancreatitis. It is defined as a walled off collection of fluid that may connect with the pancreatic ductal system and persists 4 weeks beyond the episode of pancreatitis (2).
Indications for cystgastrostomy in WOPN include infection, nutritional failure, and persistent abdominal pain (3). Walled off pancreatic necrosis also requires combined pancreatic debridement with cystgastrostomy (3). While the natural progression of pancreatic pseudocysts is resolution, a persistent symptomatic pancreatic pseudocyst is an indication for intervention. It is estimated that 40% of pancreatic pseudocysts resolve within 6 weeks. Persistence for 12 weeks or longer is associated with a low likelihood of resolution. Intervention should be performed when the cyst or cavity wall is mature, which typically takes 6 weeks (1,3).
Cystgastrostomy can be performed endoscopically or surgically (3). Surgical management is associated with decreased re-interventions but longer length of stay (4-6). Surgical management can be combined with cholecystectomy in many cases for patients with gallstone pancreatitis, further reducing the number of procedures needed (4). Minimally invasive approaches to cystgastrostomy can be performed either laparoscopically or robotically, but there are few descriptions of robotic approaches in the literature (7). We describe a robotic approach for transgastric pancreatic debridement and cystgastrostomy using the Da Vinci Xi® (Intuitive, Sunnyvale, CA, USA).
Case presentation
All procedures performed in this study were in accordance with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Patient selection and work-up
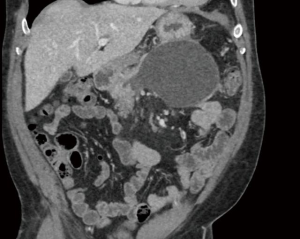
A 45-year-old male with a history of recurrent alcoholic pancreatitis presented with epigastric pain and fullness, decreased appetite and oral intake, and weight loss 8 weeks after an episode of necrotizing pancreatitis. His vital signs and laboratory values were unremarkable. A computed tomography (CT) scan of the abdomen and pelvis was performed that demonstrated a 6 cm × 7 cm area of WOPN (Figure 1). The patient underwent robotic transgastric pancreatic debridement and cystgastrostomy (Figure 2).
Equipment preference card
Here is a list of the equipment used during the procedure:
- Dual console Da Vinci Xi® (Intuitive, Sunnyvale, CA, USA);
- Laparoscopic ultrasound probe;
- 12-mm laparoscopic trocar;
- Monopolar scissors (Intuitive, Sunnyvale, CA, USA);
- Two Caudie graspers (Intuitive, Sunnyvale, CA, USA);
- Laparoscopic suction;
- Da Vinci Stapler® with two 45-mm green loads (Intuitive, Sunnyvale, CA, USA);
- 3-0 polydioxanone V-LocTM suture (Medtronic, Minneapolis, MN, USA);
- 2-0 silk suture;
- Laparoscopic retrieval bag;
- 0-vicryl UR-6 stitch (Ethicon, Somerville, NJ, USA).
Surgical procedure
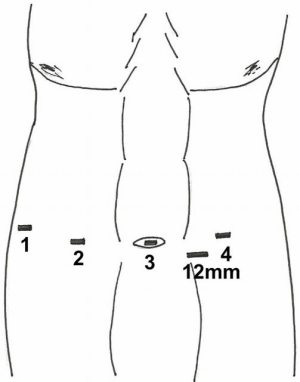
Four ports are used with the Da Vinci Xi® (Intuitive, Sunnyvale, CA, USA). The ports are placed in a similar position to robotic cholecystectomy (Figure 3). The port for arm 3 is placed through the umbilicus. Three additional ports are placed in line with this first port with one port on the patient’s left and two ports equally spaced on the patient’s right. The ports are spaced 8 cm apart. Arm 4 goes through the left-sided port, and arms 1 and 2 through the two right-sided ports. Arm 2, placed through the medial right-sided port, is used for camera placement and targeting. Two Caudie graspers are placed in arm 1 and 4. A monopolar scissors in placed in arm 3. An additional laparoscopic 12-mm trocar is placed between arms 3 and 4 for use of the suction irrigator, ultrasound probe, and laparoscopic bag.
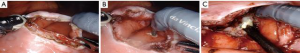
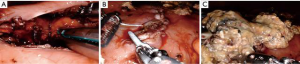
A 60cm anterior gastrostomy is made with the monopolar scissors. The Caudie graspers are used to hold open the anterior gastrostomy. The laparoscopic ultrasound is used to localize the cyst or cavity. Monopolar scissors are used to make a small 1–2-cm incision under ultrasound guidance through the posterior stomach and cyst wall. Any fluid that is expressed is suctioned out (Figure 4). A cystgastrostomy between the posterior stomach wall and the cyst wall is then created with a robotic stapler. Typically, one or two loads of the green 45-mm stapler are used depending on the size of the cavity or cyst. Necrosectomy is then performed through this cystgastrostomy. A nasogastric tube is positioned through the cystgastrostomy into the cyst cavity. The anterior gastrostomy is then closed with a running absorbable, barbed suture (3-0 polydioxanone V-LocTM suture; Medtronic, Minneapolis, MN, USA). A second layer of 2-0 silk suture is used for double layer closure of the anterior gastrostomy. All necrotic material is collected and removed via a laparoscopic bag (Figure 4). A robotic cholecystectomy can be performed via the same ports in cases of gallstone pancreatitis.
The 12-mm laparoscopic port is closed with a 0-vicryl UR-6 stitch (Ethicon, Somerville, NJ, USA). The procedure is visually depicted in Video 1.
Role of team members
The team consisted of a hepatobiliary surgeon, hepatobiliary surgical fellow, surgical scrub nurse, surgical circulating nurse, certified registered nurse anesthetist, and attending anesthesiologist. A dual console robot was used so that the attending hepatobiliary surgeon could guide the hepatobiliary surgical fellow through portions of the surgical procedure. The surgical scrub nurse was at the bedside of the patient and exchanged robotic instruments as needed.
Post-operative management
The nasogastric tube is left in place post-operatively and removed on post-operative day (POD) 1 with subsequent advancement of diet. An enhanced recovery protocol is employed with anticipation for discharge by POD 1–2. Patients are routinely seen in the clinic 2 weeks post-operatively, and repeat imaging is only obtained for recurrence of symptoms or signs of infection.
Tips, tricks, and pitfalls
- Wait at least 6–8 weeks for the cyst wall to mature prior to performing cystgastrostomy;
- An additional 12-mm laparoscopic port allows use of the laparoscopic suction, retrieval bag, and ultrasound device. This port should be placed between arms 3 and 4 to minimize collisions with the robotic instruments;
- Laparoscopic ultrasound allows accurate localization of the cyst;
- Caudie graspers are used for retracting the stomach and debriding the pancreas as they have the gentlest grip strength of the robotic graspers, minimizing trauma to the tissues;
- The described trocar placement allows for cholecystectomy during the same procedure without re-arrangement of the ports in cases of gallstone pancreatitis.
Discussion
Robotic cystgastrostomy is a safe and feasible option for patients with symptomatic pancreatic pseudocysts and WOPN that do not resolve spontaneously. We recommend robotic cystgastrostomy for patients with extensive pancreatic necrosis that is not amenable to endoscopic retrieval or may require multiple endoscopic interventions. In addition, the range of motion and articulation of the robotic arms allows simultaneous robotic cholecystectomy and cystgastrostomy without repositioning of the ports, which is sometimes necessary in laparoscopic surgery.
In this paper, we describe a robotic cystgastrostomy using a Da Vinci Xi® (Intuitive, Sunnyvale, CA, USA) and a robotic stapler. Caudie graspers are used for retracting the stomach and debriding the pancreas as they have the gentlest grip strength of the robotic graspers, minimizing trauma to the tissues. The stapler allows for faster operating time and has not been described with the robotic approach previously. We continue to use an additional 12-mm laparoscopic port. This allows us to use a laparoscopic intra-operative ultrasound probe, laparoscopic bag for removal of necrotic debris, and the suction irrigator without removal of any of the robotic arms.
The robotic approach lends itself to an excellent pancreatic debridement making it an excellent tool to treat WOPN. The articulation of the instruments and superiority of the camera with the ability to zoom into the cavity allow more thorough investigation and debridement of the pancreas in cases of WOPN. In addition, surgical approaches have been associated with fewer interventions compared to endoscopic necrosectomy in multiple studies (4-6). Some randomized controlled trials have demonstrated equivalent efficacy between laparoscopic and endoscopic drainage for pancreatic pseudocysts and WOPN in collections with less than 30% debris; however, endoscopic and robotic cystgastrostomy have never been compared, and the benefit of the robot is likely in cases with WOPN and more extensive debris (9,10). Future studies should be directed at comparing robotic and endoscopic cystgastrostomy with transgastric debridement in cases of WOPN, particularly in cases with more extensive pancreatic necrosis. While WOPN can also be debrided retroperitoneally, this usually requires pre-existing drains and risks the development of pancreatic fistula. Thus, the robotic approach offers a minimally invasive approach with the ability for a thorough debridement that does not require drains.
Conclusions
The robotic approach allows for a combined transgastric pancreatic necrosectomy and cystgastrostomy in patients with WOPN from pancreatitis. The technique described uses intra-operative ultrasound to accurately localize the cavity and a stapled cystgastrostomy to hasten operative time. A similar technique can be used to treat pancreatic pseudocyst.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.com/article/view/10.21037/jovs.2019.12.05/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lillemoe K, Jarnagin W. Master Techniques in Surgery: Hepatobiliary and Pancreatic Surgery. Philadelphia: Wolters Kluwer, 2013.
- Foster BR, Jensen KK, Bakis G, et al. Revised Atlanta Classification for Acute Pancreatitis: A Pictorial Essay. Radiographics 2016;36:675-87. [Crossref] [PubMed]
- Jarnagin WR. Blumgart's Surgery of the Liver, Biliary Tract, and Pancreas. 6th edition. Elsevier, 2017.
- Khreiss M, Zenati M, Clifford A, et al. Cyst Gastrostomy and Necrosectomy for the Management of Sterile Walled-Off Pancreatic Necrosis: a Comparison of Minimally Invasive Surgical and Endoscopic Outcomes at a High-Volume Pancreatic Center. J Gastrointest Surg 2015;19:1441-8. [Crossref] [PubMed]
- Melman L, Azar R, Beddow K, et al. Primary and overall success rates for clinical outcomes after laparoscopic, endoscopic, and open pancreatic cystgastrostomy for pancreatic pseudocysts. Surg Endosc 2009;23:267-71. [Crossref] [PubMed]
- Redwan AA, Hamad MA, Omar MA. Pancreatic Pseudocyst Dilemma: Cumulative Multicenter Experience in Management Using Endoscopy, Laparoscopy, and Open Surgery. J Laparoendosc Adv Surg Tech A 2017;27:1022-30. [Crossref] [PubMed]
- Kirks RC Jr, Sola R Jr, Iannitti DA, et al. Robotic transgastric cystgastrostomy and pancreatic debridement in the management of pancreatic fluid collections following acute pancreatitis. J Vis Surg 2016;2:127. [Crossref] [PubMed]
- Hogen R, Aziz H, Lian T, et al. Video of a robotic cystgastrostomy and transgastric pancreatic debridement with a stapled cystgastrostomy. Asvide 2020;7:073. Available online: http://www.asvide.com/watch/33112
- Garg PK, Meena D, Babu D, et al. Endoscopic versus laparoscopic drainage of pseudocyst and walled-off necrosis following acute pancreatitis: a randomized trial. Surg Endosc 2019; [Epub ahead of print]. [PubMed]
- Varadarajulu S, Bang JY, Sutton BS, et al. Equal efficacy of endoscopic and surgical cystogastrostomy for pancreatic pseudocyst drainage in a randomized trial. Gastroenterology 2013;145:583-90.e1. [Crossref] [PubMed]
Cite this article as: Hogen R, Aziz H, Lian T, Genyk Y, Sheikh MR. Robotic cystgastrostomy and transgastric pancreatic debridement: case report. J Vis Surg 2020;6:32.


