Penetrating chest trauma
Introduction
Traumatic injuries account for more than one third of the total surgical burden of disease (1). In industrialized countries, mostly blunt forces are responsible for a plethora of different injuries. In less developed countries, penetrating trauma is predominant. Especially penetrating chest trauma, mostly due to interpersonal violence, is a major cause of death in up to 25% (2). Typical life-threatening chest injuries are tension pneumothorax, open pneumothorax, massive hemothorax or cardiac tamponade (3). Cardiac or combined thoraco-abdominal injuries, predominantly after gunshot injuries, constitute exceptional deadly injuries. Most of these patients die pre-hospital. However, some deaths are preventable, particularly injuries with a cardiac tamponade or an uncontrolled hemorrhage, thus rescue time must be kept as short as possible (4). Therefore, early diagnosis and immediate adequate treatment are urgent—time is of paramount essence! The goal is to reduce morbidity and mortality by hunting for life-threatening injuries with the help of a thorough clinical examination, diagnostic imaging (chest X-ray, focused sonography, and computed tomography), and distinct patient-oriented treatment. The “ABCDE” Advanced Trauma Life Support (ATLS) approach with a standardized rapid initial assessment and management of the injured patient is one safe way (3,5). Airway obstruction problems (A) are seldom in penetrating chest trauma, they are rather encountered after penetrating neck injuries. Life threatening injuries in penetrating chest trauma involve breathing (B) and circulatory (C) problems, such as tension pneumothorax, open pneumothorax, massive hemothorax, cardiac tamponade, and hemorrhagic shock that require immediate surgical interventions (5,6). Confrontation with a penetrating chest trauma should raise suspicion for cardiac, great vessel, hilar, pulmonary and abdominal injuries and be hunted unconditionally.
Depending on the patient’s hemodynamic status, the trauma mechanism [gunshot wound (GSW) vs. stab wound (SW)], and the location of the wound(s), the adequate treatment is predetermined and initiated. Clear algorithms are published by the Western Trauma Association, the Eastern Association for the Surgery of Trauma, and ATLS (6-8). Most penetrating chest injuries can be managed non-operative (79%), however a tube thoracostomy (18%) or sternotomy/thoracotomy (3%) are necessary in selected cases (6). A stable patient with a penetrating chest trauma can be assessed and treated non-operative in case of small pneumothorax/hemothorax and no relevant additional findings. A large pneumo- or hemothorax is usually drained with a chest tube. Hemodynamic instability, defined as systolic pressure <90 mmHg and/or a persistent tachycardia >120 beats/min, usually mandates a surgical approach. Typically, a hemodynamic unstable patient with a wound that involves the central “cardiac zone” requires a sternotomy. With wounds emerging more laterally, the trauma surgeon will perform an anterolateral thoracotomy. A pulseless patient or a patient in arrest needs to be evaluated for an emergency department thoracotomy (EDT) (7).
Distinct injuries
Patients with a penetrating chest injury typically present with some distinct injuries. A penetrating cardiac wound, a hemo-, or pneumothorax are the main injuries, which need to be addressed.
Patients with cardiac injuries
Patients with penetrating cardiac injuries often die before they arrive in the hospital. Prehospital mortality rate reaches up to 86% (9). Cardiac injuries have also a high in-hospital mortality and must be ruled out vigorously, however this can be challenging since some patients have no clinical signs except the wounds at admission. Since clinicians depend considerably on objective measurements in order to ascertain the extend and precise mechanism of trauma, clear diagnostic algorithms are critical. Patients with a cardiac injury typically present (I) without clinical signs, (II) with a Beck’s triad (distended neck veins, muffled heart sounds and low cardiac output) (III) hemodynamic unstable, or (IV) without a spontaneous circulation.
Patients without clinical signs
Trauma surgeons must be alert and have a high index of suspicion if the patient is presenting hemodynamic stable with no clinical signs. A precordial wound (“cardiac zone”) or a transmediastinal trajectory are clear hints. A very subtle sign for a cardiac injury is a discomfort or even an impossibility to lie flat. Another subtle sign is the pulsus paradoxus in case of a cardiac tamponade, originating in a larger than normal decrease in stroke volume and systolic blood pressure (>10 mmHg) during inspiration. In view of missing clinical signs, reliability on diagnostic imaging is crucial and has to be guaranteed, since missed cardiac injuries can be lethal. An ultrasound, chest X-ray, and electrocardiogram (ECG) are minimal diagnostic tools and can help find or rule out a cardiac injury.
With the use of transthoracic ultrasonography, cardiac tamponades and other occult cardiac injuries can be detected with an adequate sensitivity of 87% and low false-positive rate (9). However, in case of an undrained left hemothorax, a surgical emphysema, or a hemopericardium draining into the pleural cavity, the sensitivity is clearly diminished. Occasionally, even epicardial fat pad can be misinterpreted as pericardial effusion.
A chest X-ray, if possible in an upright position, has become a standard diagnostic work-up in almost every trauma center, when suspecting an injury to the lungs or the heart. A widened mediastinum, a pneumopericardium, or a hemopericardium can be associated with a cardiac injury or an injury to the great vessels. However, the detection especially of a hemopericardium is difficult (9). In 2013, Nicol and his team described a radiological sign, the straight left heart border (= no concavity just below the left pulmonary artery). The pericardium normally consists of 5–10 milliliters (mL) fluid between the visceral and parietal layer. A continuous increase of blood in the pericardial sac (hemopericardium) will ultimately lead to an increased intrapericardial pressure as well as a straighten pericardium. This straighten pericardium appears as a straight left heart border on upright chest X-rays. This radiological sign had a low sensitivity (40%) but a high specificity (84%) in a study with 162 patients with a possible penetrating cardiac injury (10). The presence of a straight left heart border was associated with a 3.5× higher likelihood of a hemopericardium. A possible differential diagnosis of a straight left heart border is a mitral valve disease.
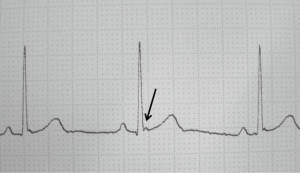
Furthermore, an occult cardiac injury can be identified with the presence of a J wave (a positive deflection on the downward slope of the R wave; Figure 1) on ECG, which sometimes is seen in patients with hypothermia as a differential diagnosis. This small notch on the downward slope of the R wave can be of additional diagnostic value and help clinicians to detect cardiac injuries with a sensitivity of 44%, specificity of 85% and a high predictive value of 91% (11).
A routine computed tomography of the chest is not always necessary, but can provide additional information for selected cases.
Stable patients with a hemo- or pneumopericardium can be treated with a subxyphoidal window and drainage to rule out active bleeding in order to avoid a sternotomy (12-14). Nevertheless, in case of an active bleeding, a sternotomy is mandatory. Needless to say, that patients without a hemopericardium need close observation on an intensive or intermediate care unit depending on associated injuries.
Patients presenting with a Beck’s triad
Patients who present with distended neck veins, muffled heart sounds and low cardiac output after a penetrating chest trauma need emergency surgery in the theatre. Depending on the clinical presentation of the wounds, a sternotomy or a thoracotomy is the ideal approach.
Patients presenting with a systolic blood pressure <90 mmHg
A hemodynamic unstable patient with a massive chest hemorrhage after a penetrating chest trauma is clinically obvious and requires immediate surgical exploration. Either via sternotomy or via thoracotomy a cardiac tamponade must first be ruled out and the underlying injury treated and second a massive intrathoracic bleeding stopped.
Patients presenting without a spontaneous circulation
A patient without spontaneous circulation or a systolic blood pressure <60 mmHg needs an EDT. The success rate of an EDT can be as high as approximately 32% (7). There are vague recommendations not to perform an EDT as long as the cardiopulmonary resuscitation has exceeded 15 minutes, because survival and neurologically intact survival is rare (8).
Patients with a hemothorax
Hemothoraces often develop after thoracic trauma, are not seldom originated by a sustained bleeding after a SW or GSW, and are associated with a high morbidity and mortality. A typical dangerous bleeding source is an intercostal artery. The quality of respiration might be reduced with diminished auscultation and percussion, although very difficult to ascertain in a noisy emergency department. Depending on the amount of blood and the speed of the active bleeding, a change of stability in the circulatory status is possible.
Whether stable or unstable, the chest X-ray is considered standard for most initial assessments of a traumatic thoracic injury. The portable single-view anteroposterior chest X-ray is fast and can screen the whole thorax without having to move the patient anywhere. A relevant traumatic hemothorax is rather easy to detect. In an upright posteroanterior chest X-ray, a pleural effusion is detected if the amount of liquid in the pleural space is between 150 to 250 mL with a typical basal lung compression (15). In supine position the effusion is moving cranially and is more difficult to ascertain, but should be detectable as soon as the effusion is more than 500 mL. Nevertheless, in left lateral position and a horizontal beam an effusion is clearly visible with a lower detection limit. Although easy to detect in this positioning, hardly ever is a patient positioned like that without the knowledge of potential additional injuries.
Modern imaging techniques are fast and sensitive. Focused assessment using Sonography in Trauma (FAST) allows for detection of a hemothorax and is more accurate than chest X-ray (8,16). Despite the widespread use of ultrasound in emergency departments, the CT scan with intravenous contrast-dye remains the most sensitive adjunct to ascertain the extend and cause of bleeding as well as to evaluate a persistent hemothorax (8). Additionally, it allows deciding which cavity needs an interventional or surgical approach. Importantly, only a stable patient or a responder to initial resuscitation is allowed to be examined with a CT scan. A possible deterioration while scanning the patient might lead to an unequivocal worse outcome due to limited equipment.
Possible short-term complications of an unceasing hemothorax are hemorrhagic shock, atelectasis and therefore reduced oxygen exchange. Ultimately, most bacterial infections are caused by hypoventilation (due to pain or external compression) or tube placement which lead to pneumonia or exacerbating empyema due to a retained hematoma. Not seldom, a fibrothorax can be observed.
The question whether to drain or not to drain is controversial. When balancing a possible life saving action against eventual long-term complications, the answer is easily done, but generally, the clinical presentation is not as obvious. Tube thoracostomy is invasive, needs some kind of anesthesia, and can remain painful throughout the hospital stay. Insertion of chest drains can be associated with malpositioning, bleeding due to an injured intercostal vein or artery, which can even be lethal, increased the length of stay, and undrained hematoma (17). Tube thoracostomy remains a controversial topic.
Generally, it can be argued that asymptomatic hemothoraces less than 300 mL (“= small”) can be treated non-operative/observational. A tube thoracostomy is done for large hemothoraces, which exceed 300 mL or correspond to a fluid level on a supine chest X-ray above the 7th rib (18). In a retrospective study, the amount of blood in the chest was a clear predictor for chest drain. In case of an additional ipsilateral flail chest the likelihood increases up to 3 times. With the presence of a pneumothorax the probability to receive a chest drain is 6 times higher (17). According to ATLS guidelines, thoracotomy is recommended in massive hemothorax (more than 1,500 mL blood after chest tube placement) or ongoing bleeding (200–400 mL per hours for the next 4 hours) (6,8). However, most surgeons depend on patient’s physiology, especially hemodynamic instability, to decide upon thoracotomy (5,8).
A persistent retained hemothorax or a persistent hemorrhage need a further work-up with a CT scan and should be considered for video-assisted thoracic surgery if greater than 300 mL (7,8).
Patients with a pneumothorax
The incidence of a pneumothorax after a penetrating chest injury is high. A pneumothorax can either be occult (only visible on CT scans), asymptomatic but visible on chest X-ray, and symptomatic. Typical symptoms are dyspnea, tachypnea, low oxygen saturation, or even severe distress in case of a tension pneumothorax or open pneumothorax.
Chest X-ray is the standard initial diagnostic imaging modality. However, it misses half of all pneumothoraces. Therefore, extended FAST (eFAST) is used to detect pneumothoraces with a higher sensitivity than chest X-ray (16). Typically, lung sliding and comet tail artefacts are missing in case of a pneumothorax (19). The highest standard remains however a chest CT, where even occult and small pneumothoraces are visible.
For a long time, placing a tube thoracostomy has been the treatment of choice for almost all radiographically detected pneumothoraces. All of us were taught, that a pneumothorax can always aggravate to a tension pneumothorax and must therefore be drained. This originates from the chest X-ray era. Due to the widespread use of CT scans, more asymptomatic pneumothoraces are detected. The question is whether to drain or not occult and small, asymptomatic pneumothoraces. We tend to drain large pneumothoraces and observe occult or small pneumothoraces even with positive pressure ventilation (8). While some authors use 20 mm as a cut-off (small vs. large), some other trauma surgeons even conclude that 35 mm is safe for observing a pneumothorax in stable patients, whether or not they are mechanically ventilated (18,20-22). Tension or open pneumothorax need obviously immediate life-saving treatment.
Patients with thoraco-abdominal penetrating injuries
Confrontation with a two-cavity trauma remains one of the biggest challenges for the trauma surgeon in the resuscitation area (23). Many questions raise up immediately (24). Is an operation needed? If so, what cavity, or in the case of the chest on which side first? The complexity of the problem is widely recognized, nevertheless optimal management for each individual patient represents a challenge.
Initial assessment by means of careful scrutiny of all cavities clinically including eFAST and chest X-ray is mandatory (25). Low-dose X-ray (LODOX) can be of great help to quickly evaluate these patients (26). Chest tube output can be misleading (intraabdominal hemorrhage draining via diaphragmatic injury into the chest and the drain). One safe way in hemodynamic unstable patients is first to rule out pericardial tamponade with eFAST, which is quite sensitive and specific and then continue with free fluid in the chest or the abdomen, mostly starting with a laparotomy (23,27). Continuation with a subxyphoidal pericardial window during laparotomy (23,28) can also rule out pericardial tamponade. Delay in therapeutic measures can be lethal.
Treatment
Key to success is the right treatment for the right patient. By following clear guidelines, as demonstrated above, we can expect low mortality with a high non-operative treatment rate. Less than 20% of the trauma patients really need surgical treatment (29). Non-operative treatment in selected patients is safe and effective, especially after SW (18,29). Sufficient analgesia and chest physiotherapy are important pillars in the management of the severely injured chest patient. Observing the patient continuously in an intensive or intermediate care unit with regular blood gas analysis and chest X-ray is mandatory.
Tube thoracostomy
Indications for a tube thoracostomy are large (300 mL) or symptomatic (causing respiratory or circulatory compromise) hemo- or pneumothoraces (5).
There are two main sites to place a tube thoracostomy. (I) 4th to 6th intercostal space just anterior to the mid-axillary line, also called the Bülau approach; (II) an isolated Monaldi approach (2nd intercostal space) is also allowed in case of an isolated pneumothorax.
According to the Hagen-Poiseuille law a chest drain with a larger diameter was used to better drain the hemothoraces with lesser clotting. Studies, however, could not find any advantages of a larger size and more (instead of one) drains (30,31). Less and smaller (28 Fr) drains are probably better due to less complications (32). Peri-interventional prophylactic antibiotics can reduce the rate of pneumonia (33).
Subxyphoidal window
Subxyphoidal window is a safe and easy way to exclude occult cardiac injury after penetrating trauma (28) (Video 1). Indication is a hemodynamic stable patient with a hemopericardium (9,14) (Video 2).
EDT
All hospitals should have clear guidelines when and where to perform an EDT, otherwise harm for patients and health care workers are possible. Indications for EDT are:
- Witnessed [less than 15 minutes of CPR (36)] or imminent cardiac arrest after penetrating chest injury;
- Severe shock [systolic blood pressure <60 mmHg (37)] after penetrating chest injury;
- Life-threatening pericardial tamponade.
The success of EDT is approximately 35% for patients with penetrating cardiac wounds, for all patients with penetrating wounds up to 15%. Contrarily, patient outcome is relatively poor for blunt trauma with 2% survival for patients in shock and less than 1% survival for patients with no vital signs (7,8).
Before EDT, bilateral chest drains should be done first to rule out tension pneumothoraces (38). If patients are not significantly improving, a thoracotomy, mostly left anterolateral thoracotomy in the 5th intercostal space (rib space below the nipple in men and in the inframammary fold in women) is the next step (5). This standard incision allows access to the pericardium (release of a pericardial tamponade), the heart (open cardiac massage and repair of cardiac injuries), the left hemithorax (lung/intercostal vessels repair), and the descending aorta (aortic cross-clamping). If necessary, the incision can be extended to the other side, a so-called clamshell incision. This incision allows rapid and efficient access to all thoracic structures as shown in an anatomic study (39).
Outcome
Outcome after penetrating chest trauma depends on several factors. The trauma mechanism (SW better than GSW), the location of the wounds (lateral better than central), additional abdominal injuries, pre-admission hypotension or even arrest, appropriate imaging and patient-oriented treatment in a timely fashion are the most important predictors (40). Thanks to a multi- and interdisciplinary approach as well as maximal synergy, patient care is optimized, which ultimately leads to a reduced morbidity and increases patient’s survival. Involvement of medical service personnel (including anesthetists) in the prehospital setting are of paramount importance since rescue time must be kept as short as possible. By means of standardized assessing the patient, conduction of the primary survey and start with lifesaving interventions first, a high percentage of patient can be saved. At the emergency department, the division of tasks between various subspecialties, including the management of non-surgical and surgical interventions, depending on physiology and injury considerations, as well as the timing of it determines outcome. Pain specialists and physiotherapists accelerate the post-injury rehabilitation course by giving a necessary framework.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Marco Scarci and Fabrizio Minervini) for the series “Chest Wall Traumas” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.com/article/view/10.21037/jovs.2019.10.03/coif). The series “Chest Wall Traumas” was commissioned by the editorial office without any funding or sponsorship. VN reports personal fees from Stryker, personal fees from Synthes, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the Helsinki Declaration (as revised in 2013). The manuscript is waived from patient informed consent according to the ethics committee or institutional review board.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Meara JG, Leather AJ, Hagander L, et al. Global Surgery 2030: evidence and solutions for achieving health, welfare, and economic development. Lancet 2015;386:569-624. [Crossref] [PubMed]
- Ekpe EE, Eyo C. Determinants of mortality in chest trauma patients. Niger J Surg 2014;20:30-4. [PubMed]
- Platz JJ, Fabricant L, Norotsky M. Thoracic Trauma: Injuries, Evaluation, and Treatment. Surg Clin North Am 2017;97:783-99. [Crossref] [PubMed]
- Pfeifer R, Halvachizadeh S, Schick S, et al. Are Pre-hospital Trauma Deaths Preventable? A Systematic Literature Review. World J Surg 2019;43:2438-46. [Crossref] [PubMed]
- Schellenberg M, Inaba K. Critical Decisions in the Management of Thoracic Trauma. Emerg Med Clin North Am 2018;36:135-47. [Crossref] [PubMed]
- Galvagno SM Jr, Nahmias JT, Young DA. Advanced Trauma Life Support((R)) Update 2019: Management and Applications for Adults and Special Populations. Anesthesiol Clin 2019;37:13-32. [Crossref] [PubMed]
- Western Trauma Association Algorithms. Available online: https://westerntrauma.org/algorithms/algorithms.html. Accessed 26 August 2019.
- Eastern Association for the Surgery of Trauma: All guidelines Trauma. Available online: https://www.east.org/education/practice-management-guidelines/category/trauma. Accessed 26 August 2019.
- Nicol AJ, Navsaria PH, Beningfield S, et al. Screening for occult penetrating cardiac injuries. Ann Surg 2015;261:573-8. [Crossref] [PubMed]
- Nicol AJ, Navsaria PH, Beningfield S, et al. A straight left heart border: a new radiological sign of a hemopericardium. World J Surg 2014;38:211-4. [Crossref] [PubMed]
- Nicol AJ, Navsaria PH. The J-wave: a new electrocardiographic sign of an occult cardiac injury. Injury 2014;45:112-5. [Crossref] [PubMed]
- Navsaria PH, Nicol AJ. Haemopericardium in stable patients after penetrating injury: is subxiphoid pericardial window and drainage enough? A prospective study. Injury 2005;36:745-50. [Crossref] [PubMed]
- Nicol AJ, Navsaria PH, Hommes M, et al. Management of a pneumopericardium due to penetrating trauma. Injury 2014;45:1368-72. [Crossref] [PubMed]
- Nicol AJ, Navsaria PH, Hommes M, et al. Sternotomy or drainage for a hemopericardium after penetrating trauma: a randomized controlled trial. Ann Surg 2014;259:438-42. [Crossref] [PubMed]
- Blackmore CC, Black WC, Dallas RV, et al. Pleural fluid volume estimation: a chest radiograph prediction rule. Acad Radiol 1996;3:103-9. [Crossref] [PubMed]
- Zieleskiewicz L, Fresco R, Duclos G, et al. Integrating extended focused assessment with sonography for trauma (eFAST) in the initial assessment of severe trauma: Impact on the management of 756 patients. Injury 2018;49:1774-80. [Crossref] [PubMed]
- Wells BJ, Roberts DJ, Grondin S, et al. To drain or not to drain? Predictors of tube thoracostomy insertion and outcomes associated with drainage of traumatic hemothoraces. Injury 2015;46:1743-8. [Crossref] [PubMed]
- Dayananda K, Kong VY, Bruce JL, et al. A selective non-operative approach to thoracic stab wounds is safe and cost effective - a South African experience. Ann R Coll Surg Engl 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Nandipati KC, Allamaneni S, Kakarla R, et al. Extended focused assessment with sonography for trauma (EFAST) in the diagnosis of pneumothorax: experience at a community based level I trauma center. Injury 2011;42:511-4. [Crossref] [PubMed]
- Almansoori TM, Hefny AF. Observing pneumothoraces: The 35-millimeter rule is safe for both blunt and penetrating chest trauma. J Trauma Acute Care Surg 2019;87:738. [Crossref] [PubMed]
- Bou Zein Eddine S, Boyle KA, Dodgion CM, et al. Observing pneumothoraces: The 35-millimeter rule is safe for both blunt and penetrating chest trauma. J Trauma Acute Care Surg 2019;86:557-64. [Crossref] [PubMed]
- Eddine SBZ, de Moya MA. Reply to Letter: Observing Pneumothoraces: The 35 Millimeter Rule Is Safe for Both Blunt and Penetrating Chest Trauma. J Trauma Acute Care Surg 2019;87:738-9. [Crossref] [PubMed]
- Berg RJ, Karamanos E, Inaba K, et al. The persistent diagnostic challenge of thoracoabdominal stab wounds. J Trauma Acute Care Surg 2014;76:418-23. [Crossref] [PubMed]
- Asensio JA, Arroyo H Jr, Veloz W, et al. Penetrating thoracoabdominal injuries: ongoing dilemma-which cavity and when? World J Surg 2002;26:539-43. [Crossref] [PubMed]
- Berg RJ, Inaba K, Okoye O, et al. The peril of thoracoabdominal firearm trauma: 984 civilian injuries reviewed. J Trauma Acute Care Surg 2014;77:684-91. [Crossref] [PubMed]
- Beningfield S, Potgieter H, Nicol A, et al. Report on a new type of trauma full-body digital X-ray machine. Emerg Radiol 2003;10:23-9. [Crossref] [PubMed]
- Matsushima K, Khor D, Berona K, et al. Double Jeopardy in Penetrating Trauma: Get FAST, Get It Right. World J Surg 2018;42:99-106. [Crossref] [PubMed]
- Hommes M, Nicol AJ, van der Stok J, et al. Subxiphoid pericardial window to exclude occult cardiac injury after penetrating thoracoabdominal trauma. Br J Surg 2013;100:1454-8. [Crossref] [PubMed]
- Van Waes OJF, Halm JA, Van Imhoff DI, et al. Selective nonoperative management of penetrating thoracic injury. Eur J Emerg Med 2018;25:32-8. [PubMed]
- Hardin J, Strumwasser A, Grabo D, et al. Evaluation of Single- versus Dual-Tube Thoracostomy after Thoracotomy for Trauma. Am Surg 2017;83:1142-6. [PubMed]
- Inaba K, Lustenberger T, Recinos G, et al. Does size matter? A prospective analysis of 28-32 versus 36-40 French chest tube size in trauma. J Trauma Acute Care Surg 2012;72:422-7. [Crossref] [PubMed]
- Bertoglio P, Guerrera F, Viti A, et al. Chest drain and thoracotomy for chest trauma. J Thorac Dis 2019;11:S186-91. [Crossref] [PubMed]
- Bradley M, Okoye O, DuBose J, et al. Risk factors for post-traumatic pneumonia in patients with retained haemothorax: results of a prospective, observational AAST study. Injury 2013;44:1159-64. [Crossref] [PubMed]
- Birrer DL, Edu S, Nicol A, et al. A standardized subxyphoidal window procedure to exclude cardiac injuries in a hemodynamic stable patient. Asvide 2019;6:327. Available online: http://www.asvide.com/watch/33011
- Birrer DL, Edu S, Nicol A, et al. A transthoracic echo with a hemopericardium. Asvide 2019;6:328. Available online: http://www.asvide.com/watch/33013
- Moore EE, Knudson MM, Burlew CC, et al. Defining the limits of resuscitative emergency department thoracotomy: a contemporary Western Trauma Association perspective. J Trauma 2011;70:334-9. [Crossref] [PubMed]
- Burlew CC, Moore EE, Moore FA, et al. Western Trauma Association critical decisions in trauma: resuscitative thoracotomy. J Trauma Acute Care Surg 2012;73:1359-63. [Crossref] [PubMed]
- Wise D, Davies G, Coats T, et al. Emergency thoracotomy: "how to do it Emerg Med J 2005;22:22-4. [Crossref] [PubMed]
- Simms ER, Flaris AN, Franchino X, et al. Bilateral anterior thoracotomy (clamshell incision) is the ideal emergency thoracotomy incision: an anatomic study. World J Surg 2013;37:1277-85. [Crossref] [PubMed]
- Asensio JA, Ogun OA, Petrone P, et al. Penetrating cardiac injuries: predictive model for outcomes based on 2016 patients from the National Trauma Data Bank. Eur J Trauma Emerg Surg 2018;44:835-41. [Crossref] [PubMed]
Cite this article as: Birrer DL, Edu S, Nicol A, Neuhaus V. Penetrating chest trauma. J Vis Surg 2020;6:9.