
Preparation and operative setup of penile prosthesis surgery
Introduction
Erectile dysfunction (ED) is defined as the inability to obtain and/or maintain an erection firm enough for satisfactory sexual performance (1) and is a common problem affecting millions of men, with increasing prevalence by age (2-4). Medical management of ED has become increasingly prevalent with the arrival of oral phosphodiesterase type 5 inhibitors in the late 1990s (5). While this is the mainstay of treatment for most men with ED, there are many patients who, for various reasons, require an alternative therapy.
One such treatment option is the insertion of a prosthetic penile implant. The first experimental alloplastic devices were implanted in the 1950s (6) and later the original modern silicone inflatable and malleable devices were described in 1973 and 1974, respectively (7,8). These devices and the techniques utilized in their implantation have changed greatly since their advent and penile implant surgery is becoming increasingly popular. In the United States, the number of patients undergoing penile prosthesis implantation increased from 17,540 in 2000 to 22,420 in 2009 (9).
One of the major driving forces in the evolution of penile prosthesis surgery has been device infection, which can be one of the more devastating complications that necessitates removal of the entire implant. On top of advances in device technology, such as the use of InhibiZone coating in Boston Scientific devices and the use of polyvinylpyrrolidone (PVP) hydrophilic coating in Coloplast implants (6,10,11), there have also been changes of technique that have led to lower rates of infectious complications. Eid first described the “no touch” technique in 2011 (12), which showed a reduced risk of infection in inflatable penile prosthesis implantation to 0.46% (13).
Our standard method to penile prosthesis surgery is to perform the implantation through a penoscrotal incision with a “no touch” technique. In order to effectively practice this technique, there is a considerable amount of care that goes into the planning of this surgery for each patient. In this paper, we will describe our setup for this style of penile implantation. This will include describing preoperative patient preparation, equipment used, layout of the operating room, and intra-operative prep of the patient up to the initial incision and setup of the operative field (Video 1).
Preoperative preparation
Although the primary focus of this paper is on the preparation and setup for penile implantation on the day of surgery, there is attention that needs to go into preparing the patient for surgery prior their arrival at the hospital. In concordance with other studies, we start our patients on oral antibiotics two days prior to surgery. Our preference is trimethoprim-sulfamethoxazole, although others have reported the use ciprofloxacin, cephalexin, or doxycycline (13,15,16). Because there is also some evidence that suggests patients with diabetes undergoing penile implant surgery may be more prone to fungal infections (17), it is thus our preference to also prescribe fluconazole to this group starting 2 days prior to surgery. All patients have a urine culture drawn. Patients with diabetes have a HbA1c drawn, with a goal for surgery of <9% (18). Patients are instructed to wash their genitals with a Hibiclens (4% w/v chlorhexidine gluconate; Molnlycke Health Care AB, Gothenburg, Sweden) soap twice daily for 2 days prior to surgery. While there is no published data that shows this practice to be effective in reducing surgical site infections for penile prostheses, it remains a common practice among prosthetic surgeons (19-21). Additionally, there are reports in orthopedic literature that preoperative surgical wash reduces infections during knee arthroplasty (22). Finally, all patients are instructed not to shave prior to surgery in order to prevent the disruption of skin that could potentially lead to bacterial colonization.
When patients arrive in the preoperative area on the day of surgery, all are screened with a physical exam to ensure the absence of an active infection, open skin lesions, or dermatitis on the scrotum, penis, groin, or lower abdomen. American Urological Association Best Policy Statement recommends the use of two IV antibiotics prior to incision, specifically an aminoglycoside (or aztreonam) plus either a 1st or 2nd generation cephalosporin or vancomycin (23). At our institution, standard antibiotic prophylaxis is gentamicin 5 mg/kg adjusted body weight and either IV vancomycin 20 mg/kg or linezolid 600 mg given 1 hour prior to incision. The antibiotics used at other institutions may vary based on medication availability and local antimicrobiograms.
Equipment preference card
Our standard setup is designed for a “no-touch technique” with a penoscrotal approach (12,13), which is optional. Some variability would be expected if performing an infrapubic or subcoronal incision. Additionally, not all surgical equipment or brands will be available to every surgeon.
Position
- Patient is maintained supine, into a modified “frog-legged” position.
- Foam padding is placed under the patient’s feet with additional foam rolled under the knees.
- Safety straps are applied to the patient’s legs below the knee.
Prep
- Electric clippers.
- Single blade razor.
- Two separate 26 mL ChloraPrep prep sticks (2% w/v chlorhexidine gluconate and 70% v/v isopropyl alcohol; Becton, Dickinson and Company, Franklin Lakes, NJ, USA).
- Kidney basin with sterile saline.
- Hibiclens bottle.
Drapes
- “Blue sticky” utility drapes.
- Large drape over legs.
- Laparotomy drape.
- Lower extremity drape for penoscrotal and circumcising approaches.
- Ioban drape (3M, St. Paul, MN, USA) is available for cases in which a second incision for ectopic is expected.
- 3M 1012 Steri-Drape with small fenestration cut in center.
- 3M 1003 Steri-Drape Isolation (Lahey) bag for Coloplast cases.
Instruments
- Standard minor set with DeBakey forceps and Metzenbaum scissors. A second minor set is used for infected cases.
- Small Deaver and Richardson retractors.
- Lone Star (Scott) retractor (Cooper Surgical, Trumbull, CT, USA).
- Lone Star Scroto-Pak: yellow blunt elastic stay hooks ×8, blue sharp elastic stay hook x1 (Cooper Surgical).
- Hegar cavernosal dilators or Brooks cavernosal dilators (Coloplast, Humlebæk, Denmark).
- Rossello cavernotomes are available for cases of corporal fibrosis but are typically not opened (Coloplast).
- Dilamezinsert, blue disposable dilamezinsert inserter (Cooper Surgical).
- Furlow insertion tools with inserts (Boston Scientific, Marlborough, MA, USA).
- Rubber shods: suture boots applied to hemostats or mosquito clamps.
- Long blade nasal speculum.
- Foerster lung grasping clamp.
- Large basins.
Sutures/needles
- 2-0 Maxon-monofilament absorbable: on GS-22 needle ×2 (Medtronic-Covidien, Minneapolis, MN, USA).
- 3-0 Polysorb-braided absorbable: on V-20 needle (Medtronic-Covidien).
- 4-0 Biosyn-monofilament absorbable: on CV-23 needle (Medtronic-Covidien).
- 2-0 Monosof-monofilament nylon: on C-14 needle (Medtronic-Covidien).
- Keith needle ×2 (comes with dilamezinsert kit).
- 15G disposable blunt needles ×2: for filling components.
- 22G disposable blunt needles ×2: for flushing air and blood from tubing prior to making device/tubing connections.
Irrigations/other supplies
- 1,000 mL plain injectable saline.
- 1,000 mL injectable saline mixed with 500 mg vancomycin and 80 mg gentamicin ×2.
- 200 mL sterile saline mixed with 300 mg rifampin and 80 mg gentamicin for Coloplast cases.
- 30 mL 0.5% ropivacaine with 4 mg dexamethasone for local injection.
- Surgicel Fibrular 2×4 (Ethicon, Somerville, NJ, USA).
- 15 Fr round hubless Jackson-Pratt drain with 1 inch Biopatch disk (Ethicon).
- 16 French Foley catheter kit.
- Sequential compression devices (SCDs).
Dressing supplies
- Dermabond skin glue (Ethicon).
- Xeroform 1×8 strip.
- 4×4 dressing sponge.
- Kerlix gauze roll.
- Kerlix gauze fluffs ×2.
- Jock strap or mesh underwear.
Procedure setup
Although there is published data in general surgery literature showing that the use of clippers is associated with lower rates of surgical site infection (SSI) (24), the uneven and elastic skin of the scrotum makes the use of clippers more difficult. Grober et al. (25) showed that on scrotal skin, both methods were equivalent in the rates of SSI, although the use of razors was associated with less skin trauma and greater hair removal as compared to clippers. In a position statement, the Sexual Medicine Society of North America suggested that surgeons be permitted their choice of razor or clipper (26). We typically perform a combination of both techniques, first utilizing the clippers to shave down lower abdominal hair and pubic hair surrounding the penis and scrotum. Note that for cases that may require a second incision for ectopic reservoir placement, the patient is shaved to the level of the umbilicus. The penis and scrotum are then washed with Hibiclens to facilitate completion of the shaving with the razor. A basin filled with sterile saline is used to rinse hair from the razor.
Proper skin preparation is one of the more pivotal components in the setup of penile implant surgery in regards to preventing device infection. Traditionally, skin preparation for penile implant surgery was performed with a 5–10-minute scrub of a povidone-iodine-based solution (13,19,27). Alcohol-based preparations were classically eschewed, presumably to avoid urethral irritation. However, Darouiche et al. provided level 1 evidence that the use of chlorhexidine-alcohol-based skin prep is superior to povidone-iodine scrubs in preventing SSI in patients undergoing clean-contaminated surgery (28). A later randomized controlled trial showed that a 2 minute chlorhexidine scrub was superior to a 10 minute povidone-iodine scrub in eradicating skin flora at the surgical skin site before genitourinary prosthetic device implantation (29). We recommend the use of two separate 26 mL chlorhexidine-alcohol-based skin prep sticks to perform an adequate sterile scrub of the entire surgical field. There is no data in urologic literature to support a minimum or maximum scrub time, though some still recommend a 10-minute prep, even with chlorhexidine (30). Because the chlorhexidine must dry for a minimum of 3 minutes prior to sterile draping of the patient, we arbitrarily use a prep time of 3 minutes as well. A timer is utilized during the scrubbing and drying phases to ensure compliance. In an effort to minimize potential contamination of the field, the surgeon or assistant prepping the skin will hand scrub and don a sterile gown and gloves during this process. The skin is prepped from the umbilicus superiorly to the proximal thigh inferiorly. The lateral skin prep extends to the patient’s flanks and lateral thighs.
The patient is positioned supine on the operating table in a modified “frog legged” position in anticipation of a penoscrotal approach. After a sterile prep, as described above, the patient is first draped with a leg drape and several sterile towels or “blue sticky” utility drapes. Local anesthesia is given at this time. There have been multiple studies examining local anesthetic options and techniques and some have performed the penile implant surgery strictly under local anesthesia (31-34). Our preference is to perform bilateral pudendal nerve blocks using a mixture of 30 mL of 0.5% ropivacaine and 4 mg dexamethasone, giving 10 mL per side. An additional 10 mL is used later in the case for local injection at the reservoir and incision sites. While many surgeons prefer the use of bupivacaine, ropivacaine has equal duration of action and less cardiac toxicity (35,36). Additionally, Xie et al. (37) performed a prospective study comparing postoperative pain outcomes for patients undergoing penile implant surgery, divided into groups receiving bupivacaine or ropivacaine, or receiving no local anesthesia. Short-term pain control was significantly improved in medicated patients as compared to the control group, and there was no significant difference in outcomes between these medications. Other techniques of delivering local anesthesia have been described as effective, including dorsal nerve blocks, penile ring blocks, and crural blocks (31). Any of these can be performed at the surgeon’s preference, as there are no randomized controlled studies directly comparing the different techniques with one another in regards to postoperative pain control.
While there are multiple publications describing the surgical steps and equipment needed in performing penile implantation (12,13,19-21,27,30,32-34), to our knowledge, there are no publications describing the physical setup of the operating room for these procedures. Our operating room setup is designed to facilitate workflow while maintaining sterile principles (Figure 1). Two mayo stands are sterilely draped and topped with sterile blue towels. The first stand is used for passing instruments to and from the surgical field and is positioned near the patient’s feet. A separate mayo stand is placed on the surgeon’s right and stores some instruments and irrigation used during device implantation and preparation. Behind the surgeon is a non-sterile discard table for maintaining the “no touch” technique. All other instruments and equipment are kept on the back table.
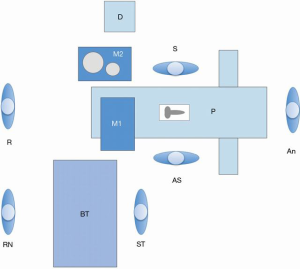
The first mayo stand is initially prepared only with the limited equipment and instruments needed to make the initial skin incision in preparation for the “no touch” technique. This includes a scalpel, two DeBakey forceps, electrocautery, and the surgical drapes. In order to utilize the “no-touch” technique, there needs to be a location for discarding these instruments that have come in contact with the skin and are considered contaminated (12,13). We utilize a surgical prep stand that is covered with a non-sterile chux pad, so that there is no confusion as to the sterility of instruments placed here. This stand is positioned near the primary surgeon so that all contaminated instruments can be placed directly onto the stand without a physical handoff to the assistant surgeon or surgical technician. The first mayo stand is utilized throughout the remainder of the case for passing sterile instruments off the field.
The surgeon’s mayo stand is specifically important for increasing operative efficiency and maintaining sterility during device implantation. On the stand are two metal basins. The larger basin/bucket is filled with 1,000 mL injectable saline mixed with 500 mg vancomycin and 80 mg gentamicin. This basin is used as the storage site for the dilators and/or dilamezinsert, blue disposable insert, Furlow instruments, and nasal speculum when not in use during the procedure. There is no published data showing that storing dilators in an antibiotic solution will decrease infection rates, but this is a technique that we would recommend, as it is simple to perform, is potentially beneficial, and carries little risk for the patient. The smaller basin is filled with sterile injectable saline for device inflation. Several syringes are pre-prepared for this purpose as well. Suture boots are applied to four hemostats or mosquito clamps to make rubber shods for the handling of device tubing.
The remainder of the equipment used during surgery is kept on the back table. Some special instruments that we would consider essential to the case are DeBakey forceps and Metzenbaum scissors for use during dissection and closure. Two Richardson retractors and a narrow Deaver retractor are commonly used for assisting with retraction; the Deaver is specifically used during reservoir placement. We have also found that a long nose nasal speculum is effective for assisting with the insertion of reservoirs into the space of Retzius. A Foerster lung grasping clamp is included with our setup, which can aid in high submuscular ectopic reservoir placement. The Crimper device is used during Boston Scientific cases for securing device tubing. Another 1,000 mL of injectable saline mixed with 500 mg vancomycin and 80 mg gentamicin is kept in a plastic basin on the back table and is used for wound irrigation throughout the case. Similar to the practice of keeping certain instruments submerged in antibiotic solution throughout the case, the clinical efficacy of local antibiotic irrigation has not been established by a prospective, randomized trial (38). In addition, there is no standardized regimen of antibiotics for wound irrigation and the duration of local antimicrobial activity is likely less than one day (39). However, we would consider this practice standard of care and recommend its regular use. For Coloplast cases, the hydrophilic PVP coating of the Titan devices reduces bacterial attachment, allows the surgeon their choice of antibiotic mixture in which to soak the implant intraoperatively, and absorbs and elutes the antibiotics in which the device is soaked (10,11). Our preference is to use 300 mg rifampin and 80 mg gentamicin mixed in 200 mL sterile saline, as a rifampin/gentamicin mixture has been shown in a meta-analysis to be more effective than other combinations (40). We prefer to keep this solution in a 3M 1003 Steri-Drape Isolation (Lahey) bag placed into a basin. Regarding our suture preference, two 2-0 Maxon or PDS sutures are used for corporotomy closure, although others may use Polysorb or Vicryl (27). A 3-0 Polysorb or Vicryl suture is used to close the Dartos fascia and the skin is closed with 4-0 Biosyn or Monocryl suture. Our standard practice is to place a surgical drain at the conclusion of the procedure, which is secured in place with a 2-0 nylon stitch.
For proper preparation and exposure of the surgical site, a Lone Star (Scott) retractor is used along with elastic stay hooks. Initially, three yellow blunt elastic hooks are used to hold open the incision and the blue sharp elastic stay hook is used on the urethra to maintain the position of the penis cephalad. We recommend the placement of a Foley catheter to empty the bladder and to aid in identification of the urethra during dissection of the corpora cavernosa and placement of corporal stay sutures. After the initial incision is made and dissection is carried down through the Dartos fascia, three elastic stay hooks are placed into the surgical wound and secured to the Lone-star retractor. At this point, the surgeons change their gloves and additional equipment from the back table is brought onto the field, including light handles for the OR lights, a new electrocautery device, handheld suction, and new instruments. The 3M 1012 Steri-Drape is used to isolate the incision from the patient’s skin. During the initial equipment setup, a small fenestration is made in the center of the drape and then marked in order to easily identify when draping. This fenestration is placed directly over the wound and additional stay hooks are used to complete exposure of the surgical site and allow for device implantation.
Conclusions
The preparation and setup for penile prosthesis surgery involves multiple steps, starting before the patient arrives at the hospital and continuing in the pre- and intraoperative settings. The choice of instruments, physical arrangement of the operating room, and surgical technique are important considerations for the surgeon performing penile prosthesis implantation, which can improve the ease of performing the surgery and improve patient outcomes.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Martin Gross, Jay Simhan and Faysal A. Yafi) for the series “Penile Prosthesis Surgery” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.com/article/view/10.21037/jovs.2019.10.02/coif). The series “Penile Prosthesis Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from all patients for publication of this article and any accompanying images/videos. A copy of each written consent is available for review by the Editors-in-Chief of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- NIH Consensus Conference. Impotence. NIH Consensus Development Panel on Impotence. JAMA 1993;270:83-90. [Crossref] [PubMed]
- Feldman HA, Goldstein I, Hatzichristou DG, et al. Impotence and its medical and psychosocial correlates: results of the Massachusetts Male Aging Study. J Urol 1994;151:54-61. [Crossref] [PubMed]
- Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA 1999;281:537-44. [Crossref] [PubMed]
- Brookes ST, Link CL, Donovan JL, et al. Relationship between lower urinary tract symptoms and erectile dysfunction: results from the Boston Area Community Health Survey. J Urol 2008;179:250-5; discussion 255. [Crossref] [PubMed]
- Goldstein I, Lue TF, Padma-Nathan H, et al. Oral sildenafil in the treatment of erectile dysfunction. Sildenafil Study Group. N Engl J Med 1998;338:1397-404. [Crossref] [PubMed]
- Le B, Burnett AL. Evolution of penile prosthetic devices. Korean J Urol 2015;56:179-86. [Crossref] [PubMed]
- Scott FB, Bradley WE, Timm GW. Management of erectile impotence. Use of implantable inflatable prosthesis. Urology 1973;2:80-2. [Crossref] [PubMed]
- Small MP, Carrion HM, Gordon JA. Small-Carrion penile prosthesis. New implant for management of impotence. Urology 1975;5:479-86. [Crossref] [PubMed]
- Montague DK. Penile prosthesis implantation in the era of medical treatment for erectile dysfunction. Urol Clin North Am 2011;38:217-25. [Crossref] [PubMed]
- Dhabuwala C, Sheth S, Zamzow B. Infection rates of rifampin/gentamicin-coated Titan Coloplast penile implants. Comparison with Inhibizone-impregnated AMS penile implants. J Sex Med 2011;8:315-20. [Crossref] [PubMed]
- Wolter CE, Hellstrom WJ. The hydrophilic-coated inflatable penile prosthesis: 1-year experience. J Sex Med 2004;1:221-4. [Crossref] [PubMed]
- Eid JF. No-touch technique. J Sex Med 2011;8:5-8. [Crossref] [PubMed]
- Eid JF, Wilson SK, Cleves M, et al. Coated implants and "no touch" surgical technique decreases risk of infection in inflatable penile prosthesis implantation to 0.46%. Urology 2012;79:1310-5. [Crossref] [PubMed]
- Szabo D, Jenkins LC. Corresponding video for preparation and operative setup of penile prosthesis surgery. Asvide 2020;7:029. Available online: http://www.asvide.com/watch/33050
- Darouiche RO, Bella AJ, Boone TB, et al. North American consensus document on infection of penile prostheses. Urology 2013;82:937-42. [Crossref] [PubMed]
- Carrasquillo RJ, Gross MS. Infection Prevention Strategies Prior to Penile Implant Surgery. Eur Urol Focus 2018;4:317-20. [Crossref] [PubMed]
- Carrasquillo RJ, Munarriz RM, Gross MS. Infection Prevention Considerations for Complex Penile Prosthesis Recipients. Curr Urol Rep 2019;20:12. [Crossref] [PubMed]
- Katz BF, Gaunay GS, Barazani Y, et al. Use of a preoperative checklist reduces risk of penile prosthesis infection. J Urol 2014;192:130-5. [Crossref] [PubMed]
- Katz DJ, Stember DS, Nelson CJ, et al. Perioperative prevention of penile prosthesis infection: practice patterns among surgeons of SMSNA and ISSM. J Sex Med 2012;9:1705-12; quiz 712-4.
- Henry GD, Mahle P, Caso J, et al. Surgical Techniques in Penoscrotal Implantation of an Inflatable Penile Prosthesis: A Guide to Increasing Patient Satisfaction and Surgeon Ease. Sex Med Rev 2015;3:36-47. [Crossref] [PubMed]
- Masterson TA, Palmer J, Dubin J, et al. Medical pre-operative considerations for patients undergoing penile implantation. Transl Androl Urol 2017;6:S824-9. [Crossref] [PubMed]
- Zywiel MG, Daley JA, Delanois RE, et al. Advance pre-operative chlorhexidine reduces the incidence of surgical site infections in knee arthroplasty. Int Orthop 2011;35:1001-6. [Crossref] [PubMed]
- Wolf JS Jr, Bennett CJ, Dmochowski RR, et al. Urologic Surgery Antimicrobial Prophylaxis Best Practice Policy Panel. Best practice policy statement on urologic surgery antimicrobial prophylaxis. J Urol 2008;179:1379-90. [Crossref] [PubMed]
- Tanner J, Norrie P, Melen K. Preoperative hair removal to reduce surgical site infection. Cochrane Database Syst Rev 2011;CD004122. [PubMed]
- Grober ED, Domes T, Fanipour M, et al. Preoperative hair removal on the male genitalia: clippers vs. razors. J Sex Med 2013;10:589-94. [Crossref] [PubMed]
- Sexual Medicine Society of North America; Position statements: Razors and Peroperative Preparation of the Male Genitalia. Available online: http://www.smsna.org/V1/about/position-statements
- Anderson PC, Jain S, Summerton DJ, et al. Surgical atlas. Insertion of an inflatable penile prosthesis. BJU Int 2007;99:467-82. [Crossref] [PubMed]
- Darouiche RO, Wall MJ Jr, Itani KM, et al. Chlorhexidine-Alcohol versus Povidone-Iodine for Surgical-Site Antisepsis. N Engl J Med 2010;362:18-26. [Crossref] [PubMed]
- Yeung LL, Grewal S, Bullock A, et al. A comparison of chlorhexidine-alcohol versus povidone-iodine for eliminating skin flora before genitourinary prosthetic surgery: a randomized controlled trial. J Urol 2013;189:136-40. [Crossref] [PubMed]
- Al-Enezi A, Al-Khadhari S, Al-Shaiji TF. Three-piece Inflatable Penile Prosthesis: Surgical Techniques and Pitfalls. J Surg Tech Case Rep 2011;3:76-83. [Crossref] [PubMed]
- Reinstatler L, Shee K, Gross MS. Pain Management in Penile Prosthetic Surgery: A Review of the Literature. Sex Med Rev 2018;6:162-9. [Crossref] [PubMed]
- Dos Reis JM, Glina S, Da Silva MF, et al. Penile prosthesis surgery with the patient under local regional anesthesia. J Urol 1993;150:1179-81. [Crossref] [PubMed]
- Ghanem H, Fouad G. Penile prosthesis surgery under local penile block anaesthesia via the infrapubic space. Int J Androl 2000;23:357-9. [Crossref] [PubMed]
- Park SS, Wilson SK, Valenzuela RJ. Subcoronal IPP can be performed under local anesthesia. Presented at: Sexual Medicine Society of North America 2015.
- Graf BM. The Cardiotoxicity of Local Anesthetics: The Place of Ropivacaine. Curr Top Med Chem 2001;1:207-14. [Crossref] [PubMed]
- Reiz S, Häggmark S, Johansson G, et al. Cardiotoxicity of ropivacaine--a new amide local anaesthetic agent. Acta Anaesthesiol Scand 1989;33:93-8. [Crossref] [PubMed]
- Xie D, Nicholson M, Azaiza M, et al. Effect of operative local anesthesia on postoperative pain outcomes of inflatable penile prosthesis: prospective comparison of two medications. Int J Impot Res 2018;30:93-6. [Crossref] [PubMed]
- Falagas ME, Vergidis PI. Irrigation with antibiotic-containing solutions for the prevention and treatment of infections. Clin Microbiol Infect 2005;11:862-7. [Crossref] [PubMed]
- Darouiche RO. Antimicrobial approaches for preventing infections associated with surgical implants. Clin Infect Dis 2003;36:1284-9. [Crossref] [PubMed]
- Mandava SH, Serefoglu EC, Freier MT, et al. Infection retardant coated inflatable penile prostheses decrease the incidence of infection: a systematic review and meta-analysis. J Urol 2012;188:1855-60. [Crossref] [PubMed]
Cite this article as: Szabo D, Jenkins LC. Preparation and operative setup of penile prosthesis surgery. J Vis Surg 2020;6:5.


