
Techniques in trileaflet aortic valve repair
Introduction
Throughout the last 20 years, significant progress in the field of aortic valve (AV) repair resulted in techniques that are achievable and now widely accepted (1) as treatment options for patients with aortic insufficiency (AI). These advances include deep understanding of the functional anatomy of the AV and pathophysiological mechanisms of AI; the development of surgical techniques to restore normal geometry to the aortic root while sparing the AV; and the maturation of the concept of functional aortic annulus (FAA) (2) as an echocardiographic and surgical concept. This understanding facilitated the development of a common language that is used by all clinicians to describe the lesions of the AV or FAA, discuss repair techniques, and compare immediate and long-term outcomes after AV repair. Our group accumulated a significant experience with AV repair techniques, over the last 20 years, and continuously refined our strategies and systematic approaches to treat different types of AV regurgitation. In this article we describe the current strategies and techniques used in our institution’s systematic approach of the regurgitant trileaflet AV.
Understanding mechanisms of AI: the AV and the FAA
Understanding the mechanism of AI is not sufficient without understanding the basic role that the FAA plays in it. The FAA (Figure 1) is the natural stent containing the AV, and that is formed by the combination of the following three structures: the aortic annulus, defined as the hinge point of the AV leaflets, the sinuses of Valsalva and the sinotubular junction (STJ) (3). This structure forms a three-dimensional cylinder where the proximal plane consists of the aortic annulus, also known as the ventriculo-aortic junction (VAJ), and the distal plane consists of the STJ. We also know that the insertion points of the AV leaflets are limited by these 2 planes, where the proximal plane, or AVJ coincides with a line connecting the base of the interleaflet triangles. This has been described in our recent publications as the basal ring (BR) (4). The distal plane, or STJ corresponds to the level of insertion of the commissures.
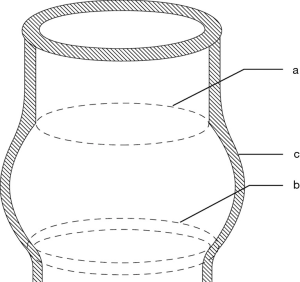
Another significant contributor in the mechanism of AI is leaflet coaptation. In a normal AV, the coaptation level of the leaflets lies approximately at the mid-level between the BR and the STJ. We also know that the height of the commissures (measured from the base of the interleaflet triangle to the top of the commissure) is equal to the external diameter of the STJ (5,6). This important element helps in determining the size of the graft in AV sparing surgery techniques.
Understanding the importance of the integrity of the FAA as well as the normal anatomy of the AV leaflets is key when aiming to achieve good valvular function. The slightest alteration to any of these components will very probably lead to AI.
Classification of AI and general principles of AV repair
In order to avoid confusion in etiologies of aortic pathologies, our institution has adopted a classification system of AI (7) that was derived from Carpentier’s classification of mitral disease, with the additional aspect including the integrity of the FAA along with the AV leaflets’ motion. In an effort to provide a common basis to be used by cardiac surgeons, cardiologists, and echocardiographers (Figure 2), the El Khoury classification regroups three major mechanisms of AI: type I AI which is caused by lesions of the FAA with normal cusp motion, type II AI which is caused by excessive cusp motion due to cusp prolapse, while type III AI is related to restrictive cusp disease.
The goal of AV repair is to restore a normal surface of coaptation by concomitantly re-establishing normal geometry both targets of repair. First, restoring normal geometry of the AV leaflets by preserving normal leaflet mobility, and second, by providing sufficient stabilisation to the BR in order to improve durability and to achieve normal anatomic dimensions of the FAA. The general principles of AV repair are as follow:
- Identifying and correcting all individual lesions leading to AI is key. Often, patients presenting with AI, are due to combined lesions of the leaflets and the FAA; for example, a dilatation of the aortic root may be associated with leaflet prolapse;
- Stabilisation of the BR is crucial. BR size is related to aortic regurgitation severity. Large BR diameter left unreduced or insufficiently stabilized during aortic valve repair can favour recurrent aortic regurgitation (8);
- Last but not least, restoration of AV leaflet mobility. The mobility of the valve leaflets is directly related to an adequate ratio between the free margin length on one hand, and the length of its base of on the annulus on the other hand.
Surgical techniques
Aortic valve reimplantation in trileaflet aortic valve repair
Originally described by David and Feindel (9) in 1993, the modified technique of aortic valve reimplantation is our preferred sparing procedure option in case of AI associated with root pathology, with or without significant aortic leaflet disease. The procedure allows BR stabilization, elimination of aortic root-associated pathology and is associated with excellent short and long-term results (10-12). Our standard procedure is outlined in Figure 3. After classic cardiopulmonary bypass, and full median sternotomy, a horizontal aortotomy is performed 1 cm above the STJ. Traction stitches (4-0 polypropylene) are placed at the tip of each of the three commissures, and are maintained on artery forceps. This allows assessment of the valve coaptation and exposure of the aortic root during external dissection. Root dissection is performed as low as possible, down to the level of the BR except in the area of the membranous septum where external root dissection is limited by the roof of the right atrium. A Gelweave Valsalva® graft (Vascutek, Terumo Company, Renfrewshire, Scotland) is used to replace the aortic root. Sizing of the graft is done by measuring the height of the commissure between the noncoronary and the left coronary sinuses. Twelve 2-0 Ticron sutures with pledgets are generally used for proximal suture line, and are placed along the fibrous portion of the aortic annulus, in a clockwise direction starting with the midportion of the interleaflet triangle between the non-coronary and the left coronary leaflet. Along the non-fibrous portions of the annulus however, where the external dissection of the aortic root is limited by muscle, these sutures are inserted along the lowest portion of the freely dissected aortic root making the proximal suture line slightly higher at that level. The prosthesis is then tied down, while simultaneously pulling downwards on the base of the prosthesis and upwards on its tip, in order to avoid distorting the AV root underneath the graft. Next, the commissures are reimplanted, at the level of the neo-STJ using 4-0 polypropylene stay sutures while pulling up on both the graft and the native commissures, which are then tied into place. Radial and horizontal traction is then applied on two adjacent commissural sutures and the distal suture line is performed in small regular steps passing the suture from outside the prosthesis to the inside. While performing the distal suture line, it is very important to consider passing through the aortic wall when passing the stitches from outside to inside, and to exclude the aortic wall when passing the stitches from inside towards the outside of the graft. This consideration helps reinforce hemostasis. At this point, the valve is tested, and the leaflets are assessed for any residual or induced prolapse. Symmetry, height and depth of coaptation are equally examined at this stage. Leaflet prolapse is managed according to the techniques described further down. The coronary buttons are then reimplanted using a running suture of 5-0 polypropylene. During this step, the suture will include a large patch of coronary tissue on the posterior versant of the anastomosis, gradually reducing the thickness of coronary rim as we approach the anterior versant of the anastomosis. Finally, the distal aortic anastomosis is performed at the level of normal aorta, after having resected any residual diseased aorta, using two 4-0 polypropylene running sutures. A Dacron ring is used to wrap the distal anastomosis when the native aorta is deemed fragile.

Sub-commissural annuloplasty
It is well reported that in bicuspid AV, sub-commissural annuloplasty is associated with questionable valve repair durability (8) which led us to change our strategy in the last years, extending the indication of valve-sparing reimplantation techniques to patients with BR >28 mm. In the trileaflet AV setting, however, the indication for valve-sparing reimplantation is less common in absence of annular dilatation. Moreover, as is the case in bicuspid AV, a large BR size was found to be a predictor or AI recurrence (4). Consequently, the non-circumferential stitch-based sub-commissural annuloplasty becomes an option in those patients where a valve-sparing reimplantation procedure is not an option, due to associated morbidity, poor prognosis or when the presence of good quality of tissue precludes future dilatation of the BR. Subcommissural annuloplasty is performed using a pledgeted Ethibond® 2/0 (Johnson and Johnson medical NV/SA) at the level of the interleaflet triangle as reported in Figure 4.

Leaflet repair in trileaflet aortic valve
Leaflet prolapse in trileaflet aortic valve leaflet prolapse occurs as the result of an increase in the free margin length compared to the length of the leaflet insertion. This leads to a decrease in the height of the prolapsing leaflet compared to the normal leaflet. Such prolapse can be present as an isolated lesion in dystrophic AI or could be induced due to the reimplantation of the valve. To correct prolapse, reducing the free margin length is needed. The amount of reduction is assessed either by eyeballing and aligning the AV leaflets, or by measuring the effective height using the Schafer’s caliper (15,16).
Two techniques are used when correcting prolapse including free margin plication and free margin resuspension.
Free margin plication
Free margin plication is our preferred approach for repair of leaflet prolapse (Figure 5). The first step for all aortic leaflet repair is to establish a reference point on which the free margin of the prolapsing leaflet must be aligned. To accomplish this, a 6-0 or 7-0 polypropylene suture is passed through the mid-point of the free margin of the non-prolapsing reference leaflet and gentle axial traction is applied. The prolapsing free margin is gently pulled parallel to the reference point of the non-prolapsing leaflet and to one side with forceps. A 5-0 or 6-0 polypropylene suture is passed through the prolapsing leaflet, from the aortic to ventricular side, at the point of intersection with the central reference suture. The prolapsing cusp is then pulled in the opposite direction and the same suture is passed from the ventricular to the aortic side of the cusp where it meets the reference point. The suture is then tied, by maintaining the fold of excess tissue on the aortic side of the leaflet. When this fold is slight excessive, the plication is then extended towards the belly of the leaflet, using a running locked 6-0 polypropylene suture. Attention must be paid in avoiding overextending the plication, in order to prevent restrictive leaflet motion. In the rare cases where the excessive tissue creates a bulging structure on the aortic versant, a triangular resection of the excess tissue is an option before performing the locked running suture.

Free margin resuspension
This technique is indicated in the setting of a fragile free margin, in the presence of multiple fenestrations of the free margin and when there is a paucity of tissue such that a plication would restrict leaflet motion. Identification of the reference point is performed in the same manner as for free margin plication. A 7-0 polytetrafluoroethylene (PTFE) suture is passed twice through the aortic wall, locking each time, at the apex of one commissure of the prolapsing leaflet. Both ends of the sutures are then run continuously over the length of the free margin, one after the other, which are then in turn tied at the apex of the opposite commissure, with two locked stitches. To reduce the length of the free margin, the leaflet is grasped at the mid-point of the free margin with forceps, and gentle traction is then applied to each arm of the PTFE sutures at one commissure. The sutures are tightened to plicate the free margin until it aligns to the adjacent reference leaflet free margin. The identical manoeuvre is then repeated at the opposite commissure for the second half of the free margin. This two-step technique allows symmetric and homogenous shortening. The two suture ends are subsequently tied and secured at each commissure (Figure 6).

Summary
AV repair is the first surgical option in patients with AI. A thorough understanding of anatomy, physiopathology and dynamic interaction between the different elements of aortic root and the AV led to refining the repair techniques. In regurgitant trileaflet AV the reimplantation procedure is the first choice especially in presence of dilated BR. However, in some cases the stabilisation of the BR can be provided with subcommissural annuloplasty when adequate tissue quality does not predict future dilatation of the BR. Two principal techniques are used to manage the prolapsing leaflet; the free margin plication is the simplest and faster technique applicable in the majority of cases, while the use of free margin resuspension technique is indicated when poor quality of tissue or large fenestrations are present.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Filip Casselman and Johan van der Merwe) for the series “Aortic and Mitral Valve Innovative Surgery” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2019.09.01). The series “Aortic and Mitral Valve Innovative Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Falk V, Baumgartner H, Bax JJ, et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur J Cardiothorac Surg 2017;52:616-64. Erratum in: Eur J Cardiothorac Surg 2017;52:832. [Crossref] [PubMed]
- Boodhwani M, El Khoury G. Principles of aortic valve repair. J Thorac Cardiovasc Surg 2010;140:S20-2; discussion S45-51.
- Bavaria JE, Vallabhajosyula P, Komlo C, et al. Valve-sparing aortic root reimplantation and cusp repair in bicuspid aortic valve: with aortic insufficiency and root aneurysm. Ann Cardiothorac Surg 2013;2:127-9. [PubMed]
- de Kerchove L, Mastrobuoni S, Boodhwani M, et al. The role of annular dimension and annuloplasty in tricuspid aortic valve repair. Eur J Cardiothorac Surg 2016;49:428-37; discussion 437-8. [Crossref] [PubMed]
- de Kerchove L, Boodhwani M, Glineur D, et al. A new simple and objective method for graft sizing in valve-sparing root replacement using the reimplantation technique. Ann Thorac Surg 2011;92:749-51. [Crossref] [PubMed]
- De Paulis R, De Matteis GM, Nardi P, et al. Opening and closing characteristics of the aortic valve after valve-sparing procedures using a new aortic root conduit. Ann Thorac Surg 2001;72:487-94. [Crossref] [PubMed]
- Boodhwani M, de Kerchove L, Glineur D, et al. Repair-oriented classification of aortic insufficiency: impact on surgical techniques and clinical outcomes. J Thorac Cardiovasc Surg 2009;137:286-94. [Crossref] [PubMed]
- Navarra E, El Khoury G, Glineur D, et al. Effect of annulus dimension and annuloplasty on bicuspid aortic valve repair. Eur J Cardiothorac Surg 2013;44:316-22; discussion 322-3. [Crossref] [PubMed]
- David TE, Feindel CM, Bos J. Repair of the aortic valve in patients with aortic insufficiency and aortic root aneurysm. J Thorac Cardiovasc Surg 1995;109:345-51; discussion 351-2. [Crossref] [PubMed]
- Mastrobuoni S, de Kerchove L, Navarra E, et al. Long-term experience with valve-sparing reimplantation technique for the treatment of aortic aneurysm and aortic regurgitation. J Thorac Cardiovasc Surg 2019;158:14-23. [Crossref] [PubMed]
- David TE, David CM, Feindel CM, et al. Reimplantation of the aortic valve at 20 years. J Thorac Cardiovasc Surg 2017;153:232-8. [Crossref] [PubMed]
- Shrestha ML, Beckmann E, Abd Alhadi F, et al. Elective David I Procedure Has Excellent Long-Term Results: 20-Year Single-Center Experience. Ann Thorac Surg 2018;105:731-8. [Crossref] [PubMed]
- Morjan M, Tamer S, Aphram G, et al. Aortic valve reimplantation in trileaflet aortic valve repair. Asvide 2019;6:253. Available online: http://www.asvide.com/watch/32938
- Morjan M, Tamer S, Aphram G, et al. Sub-commissural annuloplasty in trileaflet aortic valve repair. Asvide 2019;6:254. Available online: http://www.asvide.com/watch/32939
- Schäfers HJ, Bierbach B, Aicher D. A new approach to the assessment of aortic cusp geometry. J Thorac Cardiovasc Surg 2006;132:436-8. [Crossref] [PubMed]
- Tamer S, Mastrobuoni S, van Dyck M, et al. Free margin length and geometric height in aortic root dilatation and leaflet prolapse: implications for aortic valve repair surgery. Eur J Cardiothorac Surg 2019; [Epub ahead of print].
- Morjan M, Tamer S, Aphram G, et al. Free margin plication. Asvide 2019;6:255. Available online: http://www.asvide.com/watch/32940
- Morjan M, Tamer S, Aphram G, et al. Free margin resuspension in trileaflet aortic valve repair. Asvide 2019;6:256. Available online: http://www.asvide.com/watch/32941
Cite this article as: Morjan M, Tamer S, Aphram G, de Kerchove L, El Khoury G. Techniques in trileaflet aortic valve repair. J Vis Surg 2019;5:75.


