Robotic Ivor Lewis esophagectomy: evolving technique to optimize outcomes
Introduction
Esophagectomy continues to remain one of the most morbid surgical procedures performed (1-4). For this reason, like most operations, minimally-invasive techniques are frequently chosen for esophagectomy. Although the data are mixed about the advantages of minimally-invasive esophagectomy (MIE) (1,4-9), it will undoubtedly be increasingly chosen by patients and surgeons. In addition, the robotic platform is increasingly selected as well. Many controversies remain when performing esophagectomy for esophageal cancer such as: the ideal conduit width, the optimal manner in which to handle the pylorus, the ideal manner and location to perform the gastric-esophageal anastomosis, and even the best platform to perform esophagectomy. Currently, the literature suggests no significant difference in survival between patients undergoing open, laparoscopic minimally-invasive (MIE) or robotic minimally-invasive (RAMIE) esophagectomy, but this may be due to surgeon expertise and experience rather than surgical approach (10,11). However, the robotic platform includes several additional technologic benefits over traditional laparoscopy including 3-D magnified visualization, wristed instruments, and precision control - which in future studies may prove decreased morbidity and increased survival in patients with esophageal cancer.
In this review, we detail our evolving technique for a completely portal, robotic Ivor Lewis esophagectomy (CPR-ILE) with intrathoracic anastomosis and expand upon our most recent clinical outcomes using this approach.
Methods: patient selection and preoperative evaluation
This is a multi-institutional review of one academic surgeon’s (RJ Cerfolio) prospective experiences on a consecutive series of patients who were scheduled for Ivor Lewis or McKeown esophagectomy for esophageal cancer from January 2011 to March 2019. Operative selection was similar to that previously reported (12-14). All patients who underwent esophagectomy during this time period were offered a robotic approach (12).
In our practice we follow the National Comprehensive Cancer Network guidelines when working up patients with suspected esophageal cancer (15). Esophagoscopy is the standard for confirmatory diagnosis in conjunction with endoscopic biopsy and staging via esophageal ultrasound (EUS). Chest and abdominal computed tomography (CT) as well as positron emission tomography (PET-CT) are also used for clinical staging. Further imaging may be warranted in patients with specific symptoms concerning for metastatic disease [i.e., abdominal magnetic resonance imaging (MRI)]. In general, all patients are assessed for physical fitness to undergo invasive surgery, which includes pulmonary function testing, frailty assessment using ECOG/WHO/Zubrod scoring (16), nutritional evaluation, and system-specific testing such as cardiac stress-testing if warranted.
Patients with low cervical, mid-esophageal or distal esophageal lesions were candidates for esophagectomy. Ivor Lewis esophagectomy was used only for distal gastro-esophageal cancers (generally for lesions >23 cm from the incisors), as more proximal lesions are not suitable for chest anastomosis. For these patients, we recommend robotic mobilization of the esophagus followed by a transhiatal dissection and cervical anastomosis.
Data collection and management was approved by the institutional review boards at The University of Alabama at Birmingham (IRB #X110112008) and New York University Langone Health (IRB #18-02501). Individual patient consent was obtained to enter patient data into the prospective database.
Definitions
The Society of Thoracic Surgeons (STS) database definitions of length of stay, anastomotic complications and other morbidities, 30-day and/or operative mortality, and 90-day mortality were used (17). Complications, including anastomotic leaks, were defined using the modified Clavien-Dindo classification for surgical complications. Complications reported were grade II or above (requiring intervention) (18). Anastomotic stricture was defined as patients suffering from dysphagia preventing the progression of eating and requiring dilation within the first 6 months postoperatively with the stricture occurring at or near the anastomosis.
Ivor Lewis robotic esophagectomy
The da Vinci® system (Intuitive Surgical Inc., Sunnyvale CA, USA) is the only FDA-approved robotic system for thoracic surgery. We have previously detailed our initial (12) and ongoing (13) experience using the da Vinci® system for robotic esophagectomy, and we currently use the Xi system.
Abdominal phase
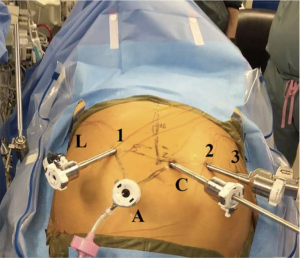
Figure 1 shows the placement of the robotic ports for the abdominal phase of the operation. While we initially preferred a laparoscopic approach for the abdominal phase, we now prefer a completely robotic approach (12) using the da Vinci® Xi robot. First, the patient is positioned supine on the operating table for esophagoscopy to confirm tumor location and assess for local invasion and/or further progression of disease. Then, both patient arms are tucked and a footboard is placed at the end of the bed. A Veress needle is used to insufflate the abdomen at Palmer’s point (2–3 cm below the left costal margin in the midclavicular line).
A 12 mm port is used to enter the abdomen in the right lower quadrant under camera guidance. The camera port is located 16 cm inferior to the xiphoid process and 3 cm to the right of the midline. The liver retractor is positioned via a right subcostal port—we prefer the Mediflex (Islandia, NY, USA) Positractor with a Lapro-Flex® self-forming retractor. Additional ports are placed laterally to the midline camera port, between 8–9 cm away from each other—2 to the patient’s left and 1 to the patient’s right as we have previously described (12,13). The right-most robotic port provides the optimal angle for robotic stapling during creation of the conduit. Our standard robotic instruments for the abdominal phase of this operation include:
- Arm 1: cadiere forceps;
- Arm 2: robotic camera;
- Arm 3: long bipolar grasper/vessel sealer;
- Arm 4: tip-up fenestrated grasper.
The patient is positioned in moderate reverse Trendelenburg and the robotic system is docked to the ports. Initial laparoscopy is performed and a snake liver retractor is inserted in the right subcostal port to lift the left lobe of the liver laterally and superiorly, exposing the diaphragmatic hiatus. If the liver surface appears cirrhotic, we perform a liver biopsy. The operation is aborted if the biopsy reveals cirrhosis, as liver failure was the most common cause of 90-day mortality in our previous series (13). Next, the gastrohepatic ligament is carefully divided with the vessel sealer to the level of the right diaphragmatic crus. Attention to aberrant anatomy in the gastrohepatic ligament is critical to avoid division of a possible replaced or accessory left hepatic artery (19). Then, the phrenoesophageal ligament is incised in order to begin circumferential dissection of the esophagus beginning at the right crus. We then mobilize and retract the lower esophagus into the mediastinum and place a circumferential Penrose drain, which can be used for retraction. It is important to avoid entering the pleural space during this maneuver and creating a capnothorax.
Next, the gastrocolic ligament is divided and a plane is established between the greater omentum and greater curvature of the stomach. Identification of the right gastroepiploic artery is critical in order to avoid injury or inadvertent division risking gastric conduit ischemia. We prefer to preserve a flap of fatty omental tissue along the greater curvature, which we use later in the thoracic phase as a buttress around the esophageal anastomosis. We have found this helps to protect the carina and right mainstem bronchus. At all times during the gastric conduit mobilization, care is taken to minimize retraction and tissue manipulation. We advise to grasp the tissue along the lesser curvature of the stomach as it will eventually be discarded. It is also important to sweep lymphatic tissue inward during the dissection to maximize lymphadenectomy in the pathologic specimen. Using this technique, our median number of lymph nodes resected was previously 18 and 22 nodes, and now approaches 25 nodes in our latest series (12,13).
Then, the short gastric arteries running along the greater curvature of the stomach are divided with the vessel sealer to the level of the left diaphragmatic crus. It is important to avoid blunt or shearing injury to the spleen in the left upper abdominal quadrant. To decrease tension on the spleen and splenic vessels, robotic arm #4 with the tip-up fenestrated grasper is used to provide lateral traction. We then divide the stomach posteriorly and use a vascular load robotic stapler to divide the left gastric artery and vein. The gastric dissection is then completed inferiorly to the pylorus using the robotic stapler, taking care to preserve the right gastroepiploic arcade. In all patients we perform a gastric drainage procedure by injection of 100 U of botulinum toxin in 4mL saline via a spinal needle into the pyloric submucosa. A pyloromyotomy or pyloroplasty may also be performed per surgeon discretion, but it is not routine in our practice as we have found botulinum toxin to be at least as equally effective, if not superior, drainage technique (20). We now also resect the lesser omentum and send as this as an additional specimen as it often contains lymph nodes. We do not routinely perform a Kocher maneuver as we feel it does not add substantial length to the gastric conduit.
The gastric conduit is then fashioned by sequential firing of the robotic stapler. The stomach is held under slight cranial-caudal tension to maximize conduit length. It is also very important to communicate with the anesthesia team to ensure that enteric devices such as temperature probes and nasogastric tubes are completely removed from the gastrointestinal tract prior to stapling. We prefer our conduit diameter to fall between 3–4 cm. The gastroesophageal junction (GEJ) is then transected just distal to the tumor—this is sent as a frozen specimen to pathology to assure a negative margin. The gastric conduit is then gently sutured to the future esophageal specimen. A soft, wide (1/2 to 1-inch) Penrose drain is sutured or stapled around the esophagus in the abdomen. Then, the diaphragmatic hiatus is opened on the right side of the chest and the gastric conduit and drain are placed gently into the right chest. We place an interrupted polypropylene or silk suture at the distal staple line to ensure it is completely pulled into the chest during the thoracic phase of the operation. The robotic instruments and ports are removed and closed.
We no longer routinely place a feeding jejunostomy tube. Instead, we use them selectively only for patients at significant risk of postoperative malnutrition. If required, the left-most abdominal port is used for access. A 12-French jejunostomy tube is placed laparoscopically or using a modified Seldinger technique. The jejunum is sutured to the abdominal wall with 2-0 polyglactin (Vicryl®, Ethicon Inc., Somerville, NJ, USA) sutures, and an anti-mesenteric serosal Witzel tunnel is created to prevent twisting or torsion of the secured small bowel.
Thoracic phase
The patient is then placed in left lateral decubitus position with the right chest facing up and tilted slightly forward, to allow the right lung and dependent blood to fall away from the posterior mediastinum. The robotic port placement for the thoracic phase is illustrated in Figure 2. The port for robotic arm #1 is marked out first, at the inferior aspect of the right axilla just slightly below the hairline and just medial to the anterior scapular edge. Next, the robotic camera port is placed 9cm inferiorly to this. The robotic camera is inserted into the chest to ensure there are no adhesions in the pleural space. We use carbon dioxide insufflation (ConMed Airseal®) at a pressure of 8-10 mmHg and a flow rate of 20 L/minute, similar to our other thoracic procedures. This insufflation assists by depressing the diaphragm and creating a larger working space in the chest. We routinely administer a paravertebral block using a 21-gauge needle filled with 0.25% bupivacaine with epinephrine which is injected posteriorly along the intercostal nerves.
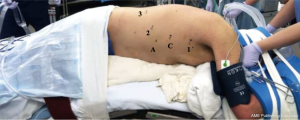
The remainder of the robotic working ports is placed under direct visualization. The port for robotic arm #1 is placed where previously marked and is used as the robotic “right hand”. The next port is placed 9cm inferior to the camera port, at the anterior axillary line, and is used for robotic arm #2. The assistant port is placed next and should be placed anterior in the chest between robotic arm #1 and the camera, taking care to avoid the rectus muscles. This port will eventually be enlarged to remove the specimen. Finally, the last port placed is for robotic arm #3, approximately 10 cm and slightly posterior from robotic arm #2. These ports are illustrated in Figure 2. Our standard robotic instruments for the thoracic phase of this operation include:
- Arm 1: tip-up fenestrated grasper;
- Arm 2: cadiere forceps;
- Arm 3: robotic camera;
- Arm 4: long bipolar grasper/vessel sealer.
The robot is again docked and the resection begins with division of the inferior pulmonary ligament and resection of regional lymph nodes as detailed in our previous work (13). We then dissect the anterior mediastinal pleura circumferentially adjacent to the esophagus, which mobilizes the esophagus from the aorta. This dissection continues down to the gastroesophageal junction (GEJ), where the Penrose drain previously placed should be identified and lifted into the chest. This Penrose drain is also helpful because it aids in exposing small aortic arterial branches, which can be clipped or ligated with bipolar cautery. We routinely divide the azygos vein with a robotic vascular staple load. Then, the esophagus is divided well above the azygos vein stump. The surgical specimen (including esophagus with tumor and the proximal stomach) are placed in a specimen bag (Anchor™, Conmed, Utica, NY, USA) and extracted via the assistant port. This port must be enlarged to extract the specimen and should then be covered with an occlusive dressing (Tegaderm™, 3M, Minneapolis, MN, USA) to maintain carbon dioxide insufflation. The proximal esophageal margin from the specimen is also sent to pathology as a frozen section to ensure adequate margins.
Real-time assessment of gastric conduit perfusion is achieved with the intravenous injection of indocyanine green contrast (ICG) and near-infrared imaging, which is incorporated in the robotic Xi camera (Firefly, Intuitive Surgical). We have found that intravenous injection of 25 mg of ICG diluted in 10 cc of sterile water effectively illuminates the tissues. The demarcation of well-perfused and non-perfused tissue helps guide an appropriate level of anastomosis, assuring adequate blood supply to the anastomosis. Malperfused or residual gastric conduit is discarded avoiding any tension of the anastomosis.
The final step of the operation is the robotic chest anastomosis. We have previously described a completely hand-sewn anastomosis (21) and an anastomosis with stapling of the posterior-aspect followed by hand-sewn anterior aspect (13) (as shown in Figure 3). We now, in the last 8 operations, have switched back to the completely hand-sewn anastomosis (illustrated in Figure 4) despite the risk of stricture, due to increased incidence of anastomotic leak.


Once the conduit is delivered into the apex of the chest using a Scanlan clamp (Scanlan International, St. Paul, MN, USA), we place it under the divided esophagus. At least 4–6 cm of the esophagus is dissected posteriorly to allow this to occur. We tack the conduit in two areas to the posterior aspect of the esophagus—this helps to line up the anastomosis. A posterior longitudinal gastrostomy is made at least 4 cm from the tip of the conduit near the greater curvature. ICG can be used to assess viability of the gastric tip. A suture-cut needle driver is placed in robotic arm 1 and a long-tip needle forceps is inserted into robotic arm 2. A row of interrupted 3-0 silk suture that is 10 cm long is placed in the seromuscular layer along the posterior wall of the anastomosis. The anterior part of the anastomosis is completed with vicryl V-Loc™ (Medtronic Inc., Minneapolis, MN, USA) sutures. First, an interior 3-0 V-Loc™ suture is placed in a running fashion to join the mucosal margins. Then, a second 3-0 V-Loc™ is placed anteriorly in a continuous Lembert fashion. The anastomosis should be buttressed using the remaining omental at pad, and the diaphragmatic hiatus is sutured closed posteriorly to prevent herniation. A single 20-French chest tube is placed apically, posterior to the anastomosis. The lung is insufflated under direct visualization, the robotic ports are removed while undergoing our “de-docking protocol”, and the incisions are closed (24).
Postoperative management
After recovering in the post-anesthesia care unit (PACU) our patients are transferred to the general surgical floor and only rarely require invasive monitoring in the intensive care unit (ICU). If a jejunostomy is placed, tube feeds begin on the first postoperative day at a rate of 10 cc/hr and is increased over the next 24–48 hours as long as the patient does not develop significant abdominal distention or ileus. Otherwise, patients are kept nil per os (NPO) until postoperative day #4 when a fiberoptic swallow study is performed. If normal, patients proceed to an esophagram with water-soluble contrast, and if no leak is observed, they are started on a clear liquid diet. Aspiration precautions are kept in place while the patient advances to a dietary consistency as tolerated. We collect serial pleural amylase levels to monitor for evidence of an esophageal leak (25) and our preferred cutoff range is 200 IU/L. The chest tube is removed once the patient’s diet or tube feeds are at goal without evidence of chylothorax. Our patients typically stay 5–8 days postoperatively (21).
Our results
Between January 2011 and March 2019, 163 consecutive patients underwent robotic esophagectomy. Table 1 shows the demographics and the clinical staging of patients. Tables 2 and 3 depict the operative outcomes, morbidity and mortality and were divided into quartiles of 50, 50, 55 and 8 patients (our most recent cohort since converting back to a completely hand-sewn anastomosis). The overall early anastomotic complication rate was 6/163 (3.7%). The 30-day mortality was 3/163, (1.8%). All deaths were secondary to respiratory complications. The 90-day mortality rate was 9/163 (5.5%). Two deaths were secondary to pneumonia, two secondary to liver failure, one urosepsis, and one unknown. As we have used process improvement, we have had no 30- or 90-day mortality in the last 62 patients. Figure 5 depicts some of the technical modifications made and changes in specific outcomes during our experience. The modifications made include: preoperative liver biopsies on select patients with a significant history of alcohol abuse (we have since denied 4 patients for surgery due to newly-diagnosed cirrhosis), stretching the gastric conduit to an increased length, using ICG to assess and confirm blood supply to the conduit (resulting in increased gastric resection in approximately 20% of patients), preserving a tongue of omentum to wrap around the anastomosis, alternating the anastomotic technique in response to patient complications, and use of postoperative chest tube amylase for early detection of leaks.
Table 1
| Characteristics | Cohort 1 (#1–50), N=50, n [%] | Cohort 2 (#51–100), N=50, n [%] | Cohort 3 (#101–155), N=55, n [%] | Cohort 4 (#156–163), N=8, n [%] | Total N=163, n [%] |
|---|---|---|---|---|---|
| Age, median [range] | 63 [47–80] | 61 [36–80] | 64 [50–78] | 70 [57–80] | 63 [36–80] |
| Gender | |||||
| Male | 44 [88] | 43 [86] | 45 [82] | 7 [88] | 139 [85] |
| Female | 6 [12] | 7 [14] | 10 [18] | 1 [12] | 24 [15] |
| Neoadjuvant chemo/radiation | 39 [78] | 39 [78] | 44 [80] | 4 [50] | 126 [77] |
| Weight loss 3 months prior to surgery (median in lbs) | 14.0 | 12.3 | 10.7 | 10.0 | 11.5 |
| Comorbidities | |||||
| Hypertension | 26 [52] | 29 [58] | 32 [58] | 4 [50] | 91 [56] |
| Coronary artery disease | 9 [18] | 7 [14] | 12 [22] | 4 [50] | 32 [20] |
| Diabetes mellitus | 8 [16] | 8 [16] | 12 [22] | 3 [38] | 31 [19] |
| COPD | 4 [8] | 4 [8] | 8 [15] | 0 [0] | 16 [10] |
| Prior cardiothoracic surgery | 2 [4] | 14 [28] | 12 [22] | 0 [0] | 28 [17] |
| FEV1 (% predicted, median) | 93.8 | 93.3 | 90.1 | 75.0 | 91.7 |
| DLCO (% predicted, median) | 86.8 | 84.0 | 88.4 | 72.0 | 85.4 |
| Hemoglobin (g/dL, median) | 13.4 | 12.7 | 13.2 | 12.7 | 12.9 |
| ECOG score (median) | 1 | 1 | 1 | 1 | 1 |
| Indication for resection | |||||
| Adenocarcinoma | 41 [82] | 42 [84] | 52 [95] | 7 [88] | 142 [87] |
| Squamous cell carcinoma | 9 [18] | 7 [14] | 3 [5] | 1 [12] | 20 [12] |
| High-grade dysplasia | 0 [0] | 1 [2] | 0 [0] | 0 [0] | 0 [0] |
| Location of tumor | |||||
| Distal esophagus or GEJ | 44 [88] | 45 [90] | 51 [93] | 3 [38] | 143 [88] |
| Mid-esophagus | 6 [12] | 5 [10] | 4 [7] | 5 [62] | 20 [12] |
| Clinical staging (by EUS) | |||||
| High-grade dysplasia | 1 [2] | 1 [2] | 0 [0] | 0 [0] | 2 [1] |
| T1bN0M0 | 2 [4] | 3 [6] | 3 [5] | 1 [12] | 9 [6] |
| T2N0M0 | 6 [12] | 6 [12] | 7 [13] | 2 [25] | 21 [13] |
| T3N0M0 | 15 [30] | 12 [24] | 17 [31] | 0 [0] | 44 [27] |
| T3N1M0 | 20 [40] | 22 [44] | 20 [36] | 1 [12] | 63 [39] |
| T3N2M0 | 4 [8] | 3 [6] | 4 [7] | 1 [12] | 12 [7] |
| T3N3M0 | 2 [4] | 2 [4] | 4 [7] | 0 [0] | 8 [5] |
| T4N2M0 | 0 [0] | 0 [0] | 0 [0] | 1 [12] | 1 [0.6] |
| TxNxM1 (by PET) | 0 [0] | 1 [2] | 0 [0] | 0 [0] | 1 [0.6] |
| T0N0M0 (complete response) | 0 [0] | 0 [0] | 0 [0] | 1 [12] | 1 [0.6] |
COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in one second; DLCO, diffusing lung carbon dioxide monoxide; ECOG, Eastern Cooperative Oncology Group; GEJ, gastroesophageal junction; EUS, endoscopic ultrasound.
Table 2
| Variable | Cohort 1 (#1–50), N=50, n [%] | Cohort 2 (#51–100), N=50, n [%] | Cohort 3 (#101–155), N=55, n [%] | Cohort 4 (#156–163), N=8, n [%] | Total N=163, n [%] |
|---|---|---|---|---|---|
| Procedure (esophagectomy) | |||||
| Ivor Lewis | 45 [90] | 50 [100] | 55 [100] | 8 [100] | 158 [97] |
| McKeown | 5 [10] | 0 [0] | 0 [0] | 0 [0] | 5 [3] |
| Operative time in minutes (median) | 361 [283–489] | 345 [281–479] | 340 [188–580] | 318 [276–354] | 343 [188–580] |
| EBL (mL, median) | 48 [20–800] | 41 [15–350] | 35 [10–650] | 20 [15–160] | 38 [10–800] |
| LOS (days, median) | 10 [5–46] | 9 [5–35] | 9 [6–23] | 9 [5–26] | 9 [5–46] |
| Blood transfusion required | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 0 [0] |
| Lymph nodes removed, median [range] | 18 [14–60] | 22 [12–50] | 20 [8–48] | 28 [19–51] | 25 [12–60] |
| R0 resection | 49 [98] | 50 [100] | 55 [100] | 8 [100] | 162 [99] |
| Morbidity | |||||
| Required ICU care from operating room | 0 [0] | 1 [2] | 0 [0] | 0 [0] | 1 [0.6] |
| Required ICU care during hospitalization | 12 [24] | 1 [2] | 0 [0] | 1 [12] | 14 [9] |
| Conversion to thoracotomy | 1 [2] | 0 [0] | 0 [0] | 0 [0] | 1 [0.6] |
| Conversion to laparotomy | 1 [2] | 0 [0] | 0 [0] | 0 [0] | 1 [0.6] |
EBL, estimated blood loss; LOS, hospital length of stay.
Table 3
| Variable | Cohort 1 [#1–50), N=50, n [%] | Cohort 2 [#51–100), N=50, n [%] | Cohort 3 [#101–155), N=55, n [%] | Cohort 4 [#156–163), N=8, n [%] | Total N=163, n [%] |
|---|---|---|---|---|---|
| Complications | |||||
| Anastomotic leak | 4 [8] | 0 [0] | 0 [0] | 2 [25] | 6 [3.7] |
| Conduit ischemia [both leaked) | 2 [4] | 0 [0] | 0 [0] | 0 [0] | 2 [1.2] |
| Chylothorax | 2 [4] | 1 [2] | 0 [0] | 0 [0] | 3 [1.8] |
| Atrial fibrillation | 6 [12] | 4 [8] | 2 [4] | 0 [0] | 12 [7.4] |
| Pneumonia or respiratory failure | 6 [12] | 0 [0] | 1 [2] | 0 [0] | 7 [4.3] |
| Reoperation in 90 days | 7 [14] | 2 [4] | 0 [0] | 1 [12.5] | 1 [0.6] |
| Bronchoscopy for secretions | 5 [10] | 2 [4] | 0 [0] | 0 [0] | 7 [4.3] |
| J-tube replacement | 2 [4] | 0 [0] | 0 [0] | 0 [0] | 2 [1.2] |
| Diaphragmatic hernia | 1 [2] | 0 [0] | 0 [0] | 0 [0] | 1 [0.6] |
| Washout and repair of anastomosis | 0 [0] | 0 [0] | 0 [0] | 1 [12.5] | 1 [0.6] |
| Mortality | |||||
| Intraoperative | 0 [0] | 0 [0] | 0 [0] | 0 [0] | 0 [0] |
| 30-day mortality | |||||
| In-hospital [suspected PE, sepsis, renal or liver failure, bowel ischemia due to embolus) | 3 [6] | 0 [0] | 0 [0] | 0 [0] | 3 [1.8] |
| 90-day mortality | |||||
| Causes: liver failure, pneumonia, urosepsis/baceriemia, and unknown | 7 [14] | 2 [4] | 0 [0] | 0 [0] | 9 [5.5] |
PE, pulmonary embolus; J-tube, jejunostomy tube.

Conclusions
Esophagectomy continues to be a morbid operation (26). The goal of minimally-invasive techniques is to add value by reducing these morbidities and improve outcomes by decreasing mortality, lowering length of stay and postoperative pain, and improving quality of life. There have been several studies that describe the different ways to perform robotic esophagectomy, including our own (5,12,13,27-32). A standardized societal nomenclature/definition consensus is needed and is currently underway to achieve fair comparisons. For now, completely portal, robotic Ivor Lewis esophagectomy (CPR-ILE) and robotic-assisted minimally-invasive esophagectomy (RAMIE) are used.
The details of our Ivor Lewis RAMIE technique are shown. The early, intra-operative, and 30 and 90-day metric outcomes are promising, and perhaps can be improved with process improvement and technical advancements. Further studies and long-term quality of life analysis is needed.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Abbas E. Abbas) for the series “Robotic Surgery for Esophageal Cancer” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: The series “Robotic Surgery for Esophageal Cancer” was commissioned by the editorial office without any funding or sponsorship. RJC serves as an unpaid editorial board member of Journal of Visualized Surgery from Feb 2018 to Jan 2020, and discloses relationships with Bovie, Community Health Services, Covidien/Medtronic, C-SATS, Davol/Bard, Ethicon, Google/Verb, Intuitive Surgical, KCI/Acelity Company, Myriad Genetics, Pinnacle, ROLO-7 Consulting Firm and TEGO Corporation. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Findlay L, Yao C, Bennett DH, et al. Non-inferiority of minimally invasive oesophagectomy: an 8-year retrospective case series. Surg Endosc 2017;31:3681-9. [Crossref] [PubMed]
- Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med 2011;364:2128-37. [Crossref] [PubMed]
- Seely AJ, Ivanovic J, Threader J, et al. Systematic classification of morbidity and mortality after thoracic surgery. Ann Thorac Surg 2010;90:936-42; discussion 942. [Crossref] [PubMed]
- Maas KW, Cuesta MA, van Berge Henegouwen MI, et al. Quality of Life and Late Complications After Minimally Invasive Compared to Open Esophagectomy: Results of a Randomized Trial. World J Surg 2015;39:1986-93. [Crossref] [PubMed]
- Xie MR, Liu CQ, Guo MF, et al. Short-term outcomes of minimally invasive Ivor-Lewis esophagectomy for esophageal cancer. Ann Thorac Surg 2014;97:1721-7. [Crossref] [PubMed]
- Yerokun BA, Sun Z, Yang CJ, et al. Minimally Invasive Versus Open Esophagectomy for Esophageal Cancer: A Population-Based Analysis. Ann Thorac Surg 2016;102:416-23. [Crossref] [PubMed]
- Luketich JD, Pennathur A, Awais O, et al. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg 2012;256:95-103. [Crossref] [PubMed]
- Biere SS, Maas KW, Bonavina L, et al. Traditional invasive vs. minimally invasive esophagectomy: a multi-center, randomized trial (TIME-trial). BMC Surg 2011;11:2. [Crossref] [PubMed]
- Rinieri P, Ouattara M, Brioude G, et al. Long-term outcome of open versus hybrid minimally invasive Ivor Lewis oesophagectomy: a propensity score matched studydagger. Eur J Cardiothorac Surg 2017;51:223-9. [PubMed]
- Weksler B, Sharma P, Moudgill N, et al. Robot-assisted minimally invasive esophagectomy is equivalent to thoracoscopic minimally invasive esophagectomy. Dis Esophagus 2012;25:403-9. [Crossref] [PubMed]
- Mitzman B, Lutfi W, Wang CH, et al. Minimally Invasive Esophagectomy Provides Equivalent Survival to Open Esophagectomy: An Analysis of the National Cancer Database. Semin Thorac Cardiovasc Surg 2017;29:244-53. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Hawn MT. Technical aspects and early results of robotic esophagectomy with chest anastomosis. J Thorac Cardiovasc Surg 2013;145:90-6. [Crossref] [PubMed]
- Cerfolio RJ, Wei B, Hawn MT, et al. Robotic Esophagectomy for Cancer: Early Results and Lessons Learned. Semin Thorac Cardiovasc Surg 2016;28:160-9. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Bass CS, et al. Fast tracking after Ivor Lewis esophagogastrectomy. Chest 2004;126:1187-94. [Crossref] [PubMed]
- Network NCC. Esophageal and Esophagogastric Junction Cancers Version 2.2018. 2018. Accessed February 19, 2019 2019.
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649-55. [Crossref] [PubMed]
- Wright CD, Kucharczuk JC, O'Brien SM, et al. Predictors of major morbidity and mortality after esophagectomy for esophageal cancer: a Society of Thoracic Surgeons General Thoracic Surgery Database risk adjustment model. J Thorac Cardiovasc Surg 2009;137:587-95; discussion 596. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Hemming AW, Finley RJ, Evans KG, et al. Esophagogastrectomy and the variant left hepatic artery. Ann Thorac Surg 1992;54:166-8. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Canon CL, et al. Is botulinum toxin injection of the pylorus during Ivor Lewis [corrected] esophagogastrectomy the optimal drainage strategy? J Thorac Cardiovasc Surg 2009;137:565-72. [Crossref] [PubMed]
- Geraci T, Cerfolio R. Robotic esophagectomy with chest anastomosis: technical aspects and clinical outcomes. Shanghai Chest 2018;2.
- Ferrari-Light D, Geraci TC, Cerfolio RJ. Robotic Ivor Lewis esophageal anastomosis using stapler for back layer. Asvide 2019;6:114. Available online: http://www.asvide.com/article/view/31113
- Ferrari-Light D, Geraci TC, Cerfolio RJ. Robotic Ivor Lewis completely hand-sewn esophageal anastomosis. Asvide 2019;6:115. Available online: http://www.asvide.com/article/view/31114
- Wei B, Cerfolio R, Hawn MT. Minimally Invasive Esophagectomy. In: Mulholland M, Albo D, Dalman R. editors. Operative Techniques In Surgery. 1st ed. Philadelphia, PA: LWW, 2014:246-53.
- Perry Y, Towe CW, Kwong J, et al. Serial Drain Amylase Can Accurately Detect Anastomotic Leak After Esophagectomy and May Facilitate Early Discharge. Ann Thorac Surg 2015;100:2041-6; discussion 2046-7. [Crossref] [PubMed]
- The Society of Thoracic Surgeons Composite Score for Evaluating Esophagectomy for Esophageal Cancer. Ann Thorac Surg 2017;103:1661-7. [Crossref] [PubMed]
- Pennathur A, Zhang J, Chen H, et al. The "best operation" for esophageal cancer? Ann Thorac Surg 2010;89:S2163-7. [Crossref] [PubMed]
- Nguyen NT, Hinojosa MW, Smith BR, et al. Minimally invasive esophagectomy: lessons learned from 104 operations. Ann Surg 2008;248:1081-91. [Crossref] [PubMed]
- Bongiolatti S, Annecchiarico M, Di Marino M, et al. Robot-sewn Ivor-Lewis anastomosis: preliminary experience and technical details. Int J Med Robot 2016;12:421-6. [Crossref] [PubMed]
- Dunn DH, Johnson EM, Morphew JA, et al. Robot-assisted transhiatal esophagectomy: a 3-year single-center experience. Dis Esophagus 2013;26:159-66. [Crossref] [PubMed]
- Wee JO, Bravo-Iniguez CE, Jaklitsch MT. Early Experience of Robot-Assisted Esophagectomy With Circular End-to-End Stapled Anastomosis. Ann Thorac Surg 2016;102:253-9. [Crossref] [PubMed]
- Okusanya OT, Sarkaria IS, Hess NR, et al. Robotic assisted minimally invasive esophagectomy (RAMIE): the University of Pittsburgh Medical Center initial experience. Ann Cardiothorac Surg 2017;6:179-85. [Crossref] [PubMed]
Cite this article as: Ferrari-Light D, Geraci TC, Cerfolio RJ. Robotic Ivor Lewis esophagectomy: evolving technique to optimize outcomes. J Vis Surg 2019;5:41.