
Laser pulmonary metastasectomy by video-assisted thoracic surgery
Introduction
The lung is the second most common site of metastasis after the liver, affecting 30% to 50% of all patients with extra-thoracic tumors (1). Carcinoma of the colon and rectum, breast, kidney and oropharynx are the most common tumors metastasizing to the lung. The International Registry of Lung Metastases retrospectively analyzed 5,206 patients with pulmonary metastasectomy from epithelial tumors, sarcoma, germ cell tumors and melanoma, and demonstrated an increased survival rate in selected patients (2). Up to date, extensive case reports and series support these results (3,4). However, biases are numerous and prospective randomized trials are lacking to validate the effectiveness of pulmonary metastasectomy compared to no treatment or compared to other treatment alternatives such as chemotherapy or stereotactic radiotherapy (5). Thus, prospective studies with control groups have been demanded by critics arguing patient selection for surgical treatment would influence survival (6,7). In response, the PulMiCC trial for patients with pulmonary metastases from colorectal carcinoma was started, in which patients are randomly assigned to pulmonary metastasectomy or active monitoring. The trial is expected to end in 2021 (8). Hence, since validated data from a prospective randomized study are not yet available, patients should be assessed in a multidisciplinary tumor board on a case-by-case basis.
Besides, criteria for selection of patients suitable for metastasectomy were stablished as early as 1965 by Thomford, and with minor modifications are valid up to this time (9): (I) the patient has adequate functional status to tolerate resection; (II) the metastases are technically resectable, (III) the primary tumor is controlled; and (IV) extrathoracic metastatic disease is absent. Additionally, there must be an absence of alternative therapy with lower morbidity (10). Over the years, parenchyma saving resection techniques and R-0 resection remained uncontroversial (11,12). Pastorino et al. (2) demonstrated a significantly more favorable 5-year survival in patients with complete resection (36%), compared to those with incomplete resection (13%). On the other hand, Casiraghi et al. (13), in contrast to Pastorino’s study, demonstrated that the number of metastastases did not affect survival in a statistically significant way. Hence, there is no consensus among thoracic surgeons whether the disease burden is an overwhelming obstacle. The decisive point would be to achieve complete resection of all sites of disease, not the absolute number of metastases per se.
In current practice it is broadly accepted that the objective of the metastasectomy is to achieve complete resection of all pulmonary metastatic tumor and to preserve as much functioning lung parenchyma as possible all the more so as redo-pulmonary metastasectomy may be required. To this end, non-anatomical lung wedge resection, widely performed by staplers, is often the preferred surgical procedure (14,15). However, for central lesions, pulmonary segmentectomy, lobectomy and eventually pulmonectomy may be required. In cases of centrally located metastases and low functional reserve in which wedge resection is not possible, or in presence of multiple metastases, the utility of laser assisted pulmonary resection and electrocautery have become increasingly popular (16,17). Cautery resection was described by Perelman in 1983 (18). This method consists of coring out the metastasis by means of coagulating the surrounding lung tissue and ligating small vessels and bronchi within the resultant cavity. Electrocautery when used in high power setting, not only generates smoke but also the carbonized tissue sticks to the tip of the cautery, which hinders its handling. Additionally, middle size vessels bleed if not properly coagulated and also, air leakage and fistula are a matter of concern because the resection surface is cauterized in an irregular manner (19). Because of above mentioned reasons, this technique has not been applicable by a thoracoscopic approach.
Pulmonary metastasectomy and laser system: background
Laser assisted pulmonary lung resection has—at least theoretically—several significant benefits. Firstly, healthy parenchyma is spared as limited lung resection is feasible in deep-located lesions. Secondly, surrounding tissues are minimally damaged or deformed by laser and thus, anatomy of adjacent structures is fully conserved. Laser also produces lung tissue shrinkage, which brings two additional advantages: mechanical reinforcement of the coagulation effect and air-tightness.
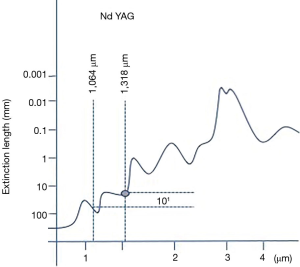
As early as 1967, Minton et al. (20) described, within an experimental setting, the use of pulsed laser energy emitted by a 1,064-nm Nd:YAG laser for resection of pulmonary metastasis in rabbit lung. In 1985, even if LoCicero et al. (21) established the 1,064-nm Nd:YAG laser for endobronchial interventions, he still favoured the CO2 laser for lung resections. Later on, CO2 was proved to be inadequate for lung surgery since CO2 is a pure cutting laser. In consequence, a number of centers in United States, Europe and Japan focused their investigation on 1,064-nm Nd:YAG laser due to its ability to vaporize and seal lung tissue simultaneously (22-27). Bare fibers and sapphire tips were used for superficial resections but failed with deep located lesions. Further research on laser wave length and lung tissue determinants aimed to develop a laser available for lung parenchyma section. On the basis of lung’s high vessel quantity, a laser with effective coagulation capability and excellent cutting property was found to be mandatory to safely resect parenchyma and consequently avoid bleeding and air leaks. Within this context, Rolle et al. (17) described that 1,318-nm wavelength provided the intended combination of effects (cutting capability plus coagulation and sealing capability) in a greatly enhanced fashion compared to 1,064-nm wavelength for lung parenchyma section. This results were achieved due to the fact that 1,318-nm Nd:YAG had a 10-fold higher absorption coefficient in water compared to the primary 1,064-nm, being capable to accomplish optimal coagulation requirements (Figure 1).
Consequently, laser assisted lung metastasectomy has gained popularity as it turned out to be convenient for resection of a high number of lung nodules, as well as for sparing parenchyma in the setting of multiple or centrally located lesions with oncologically safe margins ensuring a low risk of local recurrences.
Pulmonary metastasectomy and surgical approach
The prevailing surgical approach to pulmonary metastasectomy evolved together with surgical technologies across time, ranging from traditional open thoracotomy to VATS. There is no consensus regarding a preference for open thoracotomy over a VATS procedure, as randomized trials comparing outcomes are lacking at the time being. Available retrospective studies and meta-analyses demonstrated that VATS approach is equivalent to open surgery (28,29). However, published retrospective studies have a potential selection bias as patients with multiple nodules were more likely to consent to an open approach, offering VATS for single or few metastases. Thus, depending on surgeons’ preference and on the localization and size of the metastases, either approach has been used. Many favoured VATS for minimizing surgical trauma, enhancing postoperative recovery, and decreasing intrathoracic postoperative adhesions, which is particularly important in view of potential future repeated resections for recurrence (30,31). Others promoted an open approach to succeed in the identification of all targeted lesion via bimanual palpation, thus avoiding to overlook deep and centrally located metastases (32).
Laser assisted surgery
The theoretical benefit of laser assisted surgery is the capacity to remove a higher number of metastases due to minimal damage to surrounding tissues, while obtaining similar recurrence rates as conventional techniques. However, only few studies have reported the results for laser assisted lung resection for pulmonary metastases (Table 1). The latest studies were consistent in reporting a higher number of metastases removed and similar long-term results, compared to patients treated by other techniques. Most of the available literature is focused on LAS pulmonary metastasectomy by open thoracotomy:
Table 1
| Year | Author | Type of study | Approach, (%) | Device | Nº patients | Nº resected metastases, mean (range) |
|---|---|---|---|---|---|---|
| 2002 | Rolle | Prospective descriptive | Anterolateral thoracotomy | 1,318-nm ND:YAG | 100 | 6.3 [1–124] |
| 2006 | Rolle | Retrospective descriptive | Anteroaxillary muscle-sparing thoracotomy | 1,318-nm ND:YAG | 328 | Unilateral: 3 [1–29] |
| 2013 | Osei-Agyemangt | Retrospective cohort study; LAS |
– | 1,318-nm ND:YAG; |
301: |
LAS median: 7 |
| 2016 | Baier | Retrospective descriptive; renal cell PM | Anterolateral muscle sparing thoracotomy | 1,318-nm ND:YAG | 237 | 13 [1–110] |
| 2017 | Meyer | Prospective descriptive | VATS | 1,318-nm ND:YAG | 15 | 2 (1–4) |
| 2017 | Franzke | Retrospective cohort study LAS |
LAS [56]; thoracotomy | 1,318-nm ND:YAG; |
178: |
LAS (%), 1 PM: 46; 2 PM: 24; >2 PM: 30; |
| NLAS [44]; VATS thoracotomy | ||||||
| 2018 | Mcloughlin | Prospective descriptive | VATS | 1,320-nm ND:YAG | 7 | 1 [1–2] |
| 2018 | Porrello | Retrospective descriptive | Anteroaxillary muscle-sparing thoracotomy | 1,318-nm ND:YAG | 106 | – |
| 2018 | Schmid | Prospective cohort study LAS |
Thoracotomy for LAS | 1,320-nm ND:YAG | 106: |
LAS 6.5 [2–11]; |
PM, pulmonary metastasis; LAS, laser-assisted surgery; NLAS, non-laser assisted surgery; VATS, video-assisted thoracic surgery.
In 2002, Rolle et al. (33) described their initial experience with the 1,318-nm Nd:YAG laser for pulmonary metastasectomy in 100 patients. Subsequently, in 2006 (17), the same group published a series of 328 patients, concluding that the laser-system facilitates complete resection of multiple bilateral centrally located metastases, and consequently contributes to spare lung parenchyma.
Osei-Agyemang et al. (16) published a retrospective cohort study (n=301) comparing laser-assisted limited resection with conventional wedge or anatomic resections. A significantly higher number of resected lesions was found in the LAS group, however there was no significant correlation between the surgical technique and long-term survival.
In a retrospective study analyzing 237 patients with pulmonary metastases from renal cell carcinoma resected by 1,318-nm laser, Baier et al. (34) concluded that completeness of resection was the single most important prognostic factor for survival, even in patients with multiple metastases and unilateral single-station N1/N2 disease. In accordance with this finding, they limited the eligibility criteria for LAS pulmonary metastasectomy to functional and technical resectability.
Franzke et al. (35) reported similar overall survival for patients who underwent LAS and those operated with conventional devices. In this cohort study of 178 patients, a trend for a lower risk of local relapse was found after LAS.
Similarly, Porrello et al. (36), in a series of 106 patients, demonstrated that LAS resection of lung metastases obtained as good results as conventional surgical metastasectomy in terms of radicality of the resection and survival.
More recently, Schmid et al. (37) performed a cohort study with 106 sarcoma patients, showing that significantly more metastases were resected in the LAS group, with similar recurrence and overall survival in both groups. Limitations of this study included mixing the entities of soft-tissue and osteosarcoma, and the fact that the decision on whether to perform LAS or conventional resection was not standardized and was based on surgeon’s preference, localization, number of metastases and logistic reasons.
Laser assisted pulmonary metastasectomy by VATS
It is remarkable to notice the scarcity of studies dealing with VATS approach for laser assisted pulmonary metastasectomy. As early as in the 1990s, some thoracic surgeons published their initial experience with laser as a primary resecting tool or an adjunct to endoscopic stapling techniques to provide optimal tissue preservation by VATS. Such studies were mainly case reports or short series for resection of indeterminate nodules (38-40). At that time, 1,064-nm wavelength Nd:YAG was used, and therefore technical difficulties had yet to be solved (i.e., inefficient energy conversion into heat, higher heat dissipation and scarce penetration into tissues). In fact, it was not until 2017 that Meyer et al. (41) published the first series of 15 patients who underwent VATS laser pulmonary metastasectomy. Soon after, in 2018, McLoughlin (42) reported a series of 7 patients.
Meyer et al. concluded that VATS for pulmonary metastases is safe and effective. In this pilot study the possibility of palpating the entire lung through a minithoracotomy was highlighted. The authors claimed that systematic palpation of the whole lung could solve the shortcomings of VATS compared to open surgery. According to the authors, the potential risk of missing pulmonary nodules was decreased, even if they agreed with the fact that thanks to the improved quality of CT scans, lung metastases are less frequently overlooked. Likewise, Meyer et al. reported that via a utility minithoracotomy the laser hand piece and the smoke evacuator system could be introduced in the same manner as in open surgery. The shortness and the straight design of the laser handpiece (LIMAX 120; KLS Martin GmbH & Co KG) were identified as points to be improved for the development of VATS laser metastasectomy. They suggested to use a small, curved laser fiber holder instead of a laser handpiece as it could be guided to any part of the chest cavity—more convenient for VATS. As it was a pilot study, patients with centrally located lesions were excluded and the number of resected metastases was low (mean 2; range, 1–4).
Lastly, McLoughlin et al. reported their preliminary experience with thoracoscopic laser metastasectomy and concluded it is safe and efficient. Similar to Meyer et al., bimanual palpation of lung parenchyma was performed, and an additional smoke evacuator was inserted through the utility port. In the case of too small or deep lesions a hooked wire was placed preoperatively. This measure reduced the likelihood of conversion to open surgery. No technical complications occurred. As a preliminary study, again the number of resected metastases per patient was low (mean 1; range, 1–2).
In summary, there is a weak body of evidence concerning laser pulmonary metastasectomy by VATS. Published data considers VATS laser metastasectomy to be safe and effective. Inherent reduced lung exposure by the thoracoscopic approach seems to be solved by manual palpation through a utility minithoracotomy and/or additional ports. Failure to target small or centrally located lesions could be overcome by preoperative CT guided wire localization.
The most prominent disadvantage of using laser for lung resection is the large amount of smoke generated within the pleural space, therefore, sometimes it has to be evacuated to continue the surgery, this fact being especially tiresome and time consuming. Even if in the latest lasers a smoke evacuator is available, often a high-performance smoke evacuation system would be necessary to keep the pleural cavity clear of smoke. In that sense, advances in technology have facilitated the handling of laser, from initial short rigid laser handpiece to a bare fiber conducted through a guiding tube for endoscopic purposes.
Based on our limited experience comprising only a few patients who underwent laser metastasectomy by VATS, we conclude that for the time being, it is not a safe and recommendable procedure due to already mentioned problems (Figure 2).

Limitations for LAS include initial economic investment, staff education in laser safety including the use of protection googles, and the large amount of smoke generated in pleural cavity, hampering visualization and being time consuming.
Summary
LAS is a safe and effective technique for pulmonary metastasectomy. The available literature consists in retrospective studies and case series dealing almost exclusively with open surgery. According to these data, a higher number of metastases are removed and healthy lung parenchyma is spared, while obtaining similar recurrence rates compared to conventional staplers. In central lesions, optimal surgical margins can be achieved around the lesion avoiding injury to deep located structures such as major vessels or bronchi. Over the years, technology evolved obtaining lasers with excellent balance between cutting, hemostasis, and pneumostasis making a good tool for limited lung resections.
However, the VATS approach for pulmonary metastasectomy with the laser remains poorly spread. In fact, just two series of patients with one or two resected metastases are published. Consequently, there is no evidence to support that the postulated benefits of open laser-assisted lung metastasectomy can be achieved by VATS. At the present time and with the available technology laser lung metastasectomy does not seem to be a technique that can be recommended for general use.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Michel Gonzalez) for the series “Advancement in the Surgical Treatment of Pulmonary Metastasis” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2019.03.06). The series “Advancement in the Surgical Treatment of Pulmonary Metastasis” was commissioned by the editorial office without any funding or sponsorship. FT reports personal fees from Stryker/Novadaq (travel fees), personal fees from Medtronic (travel and consulting fees), outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Davidson RS, Nwogu CE, Brentjens MJ, Anderson TM. The surgical management of pulmonary metastasis: current concepts. Surg Oncol 2001;10:35-42. [Crossref] [PubMed]
- Pastorino U, Buyse M, Friedel G, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 1997;113:37-49. [Crossref] [PubMed]
- Chudgar NP, Brennan MF, Munhoz RR, et al. Pulmonary metastasectomy with therapeutic intent for soft-tissue sarcoma. J Thorac Cardiovasc Surg 2017;154:319-30.e1. [Crossref] [PubMed]
- Hornbech K, Ravn J, Steinbrüchel DA. Outcome after pulmonary metastasectomy: analysis of 5 years consecutive surgical resections 2002-2006. J Thorac Oncol 2011;6:1733-40. [Crossref] [PubMed]
- Treasure T, Milošević M, Fiorentino F, et al. Pulmonary metastasectomy: what is the practice and where is the evidence for effectiveness? Thorax 2014;69:946-9. [Crossref] [PubMed]
- Åberg T, Treasure T. Analysis of pulmonary metastasis as an indication for operation: an evidence-based approach. Eur J Cardiothorac Surg 2016;50:792-8. [Crossref] [PubMed]
- Aberg T, Malmberg KA, Nilsson B, et al. The effect of metastasectomy: fact or fiction? Ann Thorac Surg 1980;30:378-84. [Crossref] [PubMed]
- Treasure T, Russell C, Macbeth F. Re-launch of PulMiCC trial to discover the true effect of pulmonary metastasectomy on survival in advanced colorectal cancer. BMJ 2015;351:h6045. [Crossref] [PubMed]
- Thomford NR, Woolner LB, Clagett OT. The surgical treatment of metastatic tumors in the lungs. J Thorac Cardiovasc Surg 1965;49:357-63. [PubMed]
- Erhunmwunsee L, Tong BC. Preoperative Evaluation and Indications for Pulmonary Metastasectomy. Thorac Surg Clin 2016;26:7-12. [Crossref] [PubMed]
- McCormack PM, Martini N. The changing role of surgery for pulmonary metastases. Ann Thorac Surg 1979;28:139-45. [Crossref] [PubMed]
- Vogt-Moykopf I, Krysa S, Bülzebruck H, et al. Surgery for pulmonary metastases. The Heidelberg experience. Chest Surg Clin N Am 1994;4:85-112. [PubMed]
- Casiraghi M, De Pas T, Maisonneuve P, et al. A 10-year single-center experience on 708 lung metastasectomies: the evidence of the "international registry of lung metastases". J Thorac Oncol 2011;6:1373-8. [Crossref] [PubMed]
- Venuta F, Rolle A, Anile M, et al. Techniques used in lung metastasectomy. J Thorac Oncol 2010;5:S145-50. [Crossref] [PubMed]
- Pfannschmidt J, Egerer G, Bischof M, et al. Surgical intervention for pulmonary metastases. Dtsch Arztebl Int 2012;109:645-51. [PubMed]
- Osei-Agyemang T, Palade E, Haderthauer J, et al. Pulmonary metastasectomy: an analysis of technical and oncological outcomes in 301 patients with a focus on laser resection. Zentralbl Chir 2013;138:S45-51. [PubMed]
- Rolle A, Pereszlenyi A, Koch R, et al. Laser resection technique and results of multiple lung metastasectomies using a new 1,318 nm Nd:YAG laser system. Lasers Surg Med 2006;38:26-32. [Crossref] [PubMed]
- Cooper JD, Perelman M, Todd TR, et al. Precision cautery excision of pulmonary lesions. Ann Thorac Surg 1986;41:51-3. [Crossref] [PubMed]
- Kirschbaum A, Braun S, Rexin P, et al. Comparison of local tissue damage: monopolar cutter versus Nd:YAG laser for lung parenchyma resection. An experimental study. Interact Cardiovasc Thorac Surg 2014;18:1-6. [Crossref] [PubMed]
- Minton JP, Andrews NC, Jesseph JE. Pulsed laser energy in the management of multiple pulmonary metastases. J Thorac Cardiovasc Surg 1967;54:707-13. [PubMed]
- LoCicero J, Hartz RS, Frederiksen JW, et al. New applications of the laser in pulmonary surgery: hemostasis and sealing of air leaks. Ann Thorac Surg 1985;40:546-50. [Crossref] [PubMed]
- LoCicero J, Frederiksen JW, Hartz RS, et al. Laser-assisted parenchyma-sparing pulmonary resection. J Thorac Cardiovasc Surg 1989;97:732-6. [PubMed]
- Moghissi K, Dench M, Goebells P. Experience in non-contact Nd YAG laser in pulmonary surgery. A pilot study. Eur J Cardiothorac Surg 1988;2:87-94. [Crossref] [PubMed]
- Kodama K, Doi O, Higashiyama M, et al. Surgical management of lung metastases. Usefulness of resection with the neodymium:yttrium-aluminum-garnet laser with median sternotomy. J Thorac Cardiovasc Surg 1991;101:901-8. [PubMed]
- Branscheid D, Krysa S, Wollkopf G, et al. Does ND-YAG laser extend the indications for resection of pulmonary metastases? Eur J Cardiothorac Surg 1992;6:590-6; discussion 597. [Crossref] [PubMed]
- Kodama K, Doi O, Higashiyama M, et al. Usefulness of Nd-YAG laser for the excision of multiple lung metastases and segmentectomy for primary lung cancer. Kyobu Geka 1992;45:51-5. [PubMed]
- Mineo TC, Ambrogi V, Pompeo E, et al. The value of the Nd:YAG laser for the surgery of lung metastases in a randomized trial. Chest 1998;113:1402-7. [Crossref] [PubMed]
- Carballo M, Maish MS, Jaroszewski DE, et al. Video-assisted thoracic surgery (VATS) as a safe alternative for the resection of pulmonary metastases: a retrospective cohort study. J Cardiothorac Surg 2009;4:13. [Crossref] [PubMed]
- Nakajima J, Takamoto S, Tanaka M, et al. Thoracoscopic surgery and conventional open thoracotomy in metastatic lung cancer. Surg Endosc 2001;15:849-53. [Crossref] [PubMed]
- Gossot D, Radu C, Girard P, et al. Resection of Pulmonary Metastases From Sarcoma: Can Some Patients Benefit From a Less Invasive Approach? Ann Thorac Surg 2009;87:238-43. [Crossref] [PubMed]
- Meng D, Fu L, Wang L, et al. Video-assisted thoracoscopic surgery versus open thoracotomy in pulmonary metastasectomy: a meta-analysis of observational studies. Interact Cardiovasc Thorac Surg 2016;22:200-6. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, McCarty TP, et al. A Prospective Study to Determine the Incidence of Non-Imaged Malignant Pulmonary Nodules in Patients Who Undergo Metastasectomy by Thoracotomy With Lung Palpation. Ann Thorac Surg 2011;91:1696-700. [Crossref] [PubMed]
- Rolle A, Koch R, Alpard SK, et al. Lobe-sparing resection of multiple pulmonary metastases with a new 1318-nm Nd:YAG laser--first 100 patients. Ann Thorac Surg 2002;74:865-9. [Crossref] [PubMed]
- Baier B, Kern A, Kaderali L, et al. Retrospective survival analysis of 237 consecutive patients with multiple pulmonary metastases from advanced renal cell carcinoma exclusively resected by a 1318-nm laser. Interact Cardiovasc Thorac Surg 2015;21:211-7. [Crossref] [PubMed]
- Franzke K, Natanov R, Zinne N, et al. Pulmonary metastasectomy - A retrospective comparison of surgical outcomes after laser-assisted and conventional resection. Eur J Surg Oncol 2017;43:1357-64. [Crossref] [PubMed]
- Porrello C, Gullo R, Vaglica A, et al. Pulmonary Laser Metastasectomy by 1318-nm Neodymium-Doped Yttrium-Aluminum Garnet Laser: A Retrospective Study About Laser Metastasectomy of the Lung. Surg Innov 2018;25:142-8. [Crossref] [PubMed]
- Schmid S, Le UT, Zeisel C, et al. Pulmonary metastasectomy in sarcoma-experiences with laser-assisted resection. J Thorac Dis 2018;10:314-20. [Crossref] [PubMed]
- Landreneau RJ, Keenan RJ, Hazelrigg SR, et al. VATS wedge resection of the lung using the neodymium:yttrium-aluminum garnet laser. Ann Thorac Surg 1993;56:758-61. [Crossref] [PubMed]
- Keenan RJ, Landreneau RJ, Hazelrigg SR, et al. Video-assisted thoracic surgical resection with the neodymium:yttrium-aluminum-garnet laser. J Thorac Cardiovasc Surg 1995;110:363-7. [Crossref] [PubMed]
- Dowling RD, Wachs ME, Ferson PF, et al. Thoracoscopic neodymium: yttrium aluminum garnet laser resection of a pulmonary metastasis. Cancer 1992;70:1873-5. [Crossref] [PubMed]
- Meyer C, Bartsch D, Mirow N, et al. Video-Assisted Laser Resection of Lung Metastases-Feasibility of a New Surgical Technique. Thorac Cardiovasc Surg 2017;65:382-6. [Crossref] [PubMed]
- Mc Loughlin JB, O’Sullivan KE, Brown RH, et al. Limax Nd:YAG laser-assisted thoracoscopic resection of pulmonary metastases; a single centre’s initial experience. Ir J Med Sci 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Ojanguren A, Karenovics W, Dackam S, et al. Laser resection of a small peripheral lung metastasis by 1,318-nm Nd:YAG laser. Notice the fully coagulated and sealed surgical site and the high amount of smoke produced during the procedure. Asvide 2019;6:102. Available online: http://www.asvide.com/article/view/31000
Cite this article as: Ojanguren A, Karenovics W, Dackam S, Demarchi M, Triponez F. Laser pulmonary metastasectomy by video-assisted thoracic surgery. J Vis Surg 2019;5:40.


