Uniportal segmentectomy: an alternative for resection of deeply situated lung metastases
Introduction
Even though lung metastasectomy of different primary cancers is part of daily practice of many thoracic surgeons, there is only limited evidence concerning the benefit of such operations for the patient (1). A survey among members of the European Society of Thoracic Surgeons showed that local control at primary tumor site and possibility of complete resection of lung metastases were widely accepted prerequisites for surgery (2). Apart from that, surgical approach and extent of resection must be tailored to the individual case (3). VATS-approaches seem to offer similar oncologic outcome with less morbidity for cases with singular lesion or up to three lung metastases (4). Mastering of a wide arsenal of surgical techniques allows the thoracic surgeon to choose the optimal strategy. Multiport and especially uniportal VATS segmentectomy can help solving challenging situations.
Since there is no clear evidence that lung metastasectomy positively influences the outcome of patients, indications for surgery should be taken in multidisciplinary oncological boards. Once the two aforementioned requisites are fulfilled, i.e., local tumor control at primary site and feasibility of complete resection of lung metastases, the patient must be checked for tolerance of the planned procedure. Relative criteria influencing the decision towards surgery also comprise the length of disease-free interval after treatment of the primary tumor site as well as the number of lung metastases (5). The histology of the primary disease has an influence on the outcome, whereas colorectal cancer is the best studied entity (6). Following strict indication criteria, some centers have shown long term survival rates of almost 30% at 10 years (7).
In recent years the focus was on safety of VATS approaches compared to thoracotomy (8). The advantages of the lesser invasiveness and lower risk of adherence building preserve the possibility of the patient to undergo further metastasectomy. VATS has been criticized for the limited possibility of palpation of the lung during the procedure, leaving up to 20% of metastases unrecognized, but no difference in survival has been found so far (9,10). In an attempt to minimize trauma to the patients, even series with uniportal non-intubated approaches have been published (11).
Minimal invasive segmentectomy has much developed in recent years and metastasectomy represent up to 20% of the indications (12,13). Others before have recognized a niche for sublobar anatomic resections in metastatic lesions which cannot be resected by extra-anatomic resections and would otherwise make a lobectomy necessary (14).
Case presentation
Case 1
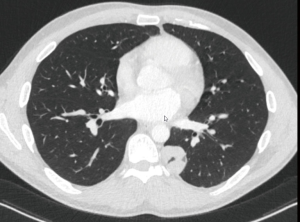
A 52-year-old patient with a history of squamous cell carcinoma of the larynx, treated by laryngectomy and neck dissection followed by sequential adjuvant radiotherapy and chemotherapy, presented one year later with a solitary lung nodule. The positron emission tomography combined with computed tomography (PET/CT) showed a 12 mm spiculated nodule in the ventral segment of the left upper lobe (S3, Figure 1) with a standardized uptake value (SUV) of 3.1. There were no signs of recurrence in the neck. The differential diagnosis includes metastasis of the head and neck cancer as well as bronchial carcinoma. Lung function tests were not possible due to previous laryngectomy, but the patient reported no limitations in daily activities and was otherwise fit for surgery. In order to allow for a complete resection of this centrally located nodule by at the same time offering an oncological sound option in case of non-small cell lung cancer, we proceeded to a uniportal S3-segmentectomy with mediastinal lymphadenectomy (Figure 2). The chest tube was removed on postoperative day (POD) 1 and the patient was discharged on POD 2. The final histology showed a 2.5 cm metastasis of the known squamous cell carcinoma of the larynx with free resection margins and 9 negative lymph nodes (Stations 5, 6, 7, 9, 10, 11, 12 and 13). The board for head and neck malignancy proposed to proceed with an adjuvant chemotherapy.

Case 2
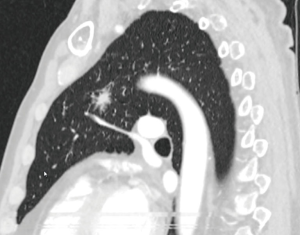
A 35-year-old male patient presented with a 5 cm mass in the left lower lobe (Figure 3). Three years previously he had undergone left orchidectomy followed by chemotherapy for a mixed form of a testicular tumor (seminoma and teratoma). The CT-scan showed the lesion in the posterobasal segment (S10) of the left lower lobe with no other signs of tumor manifestation. Lung function tests were normal with a forced expiratory volume in the first second (FEV1) of 117%. Upon discussion in the board for thoracic malignancy we proceeded to the S10-segmentectomy with en-bloc resection of lymph node station 8 and mediastinal lymphadenectomy (Figure 4). The chest tube was removed on POD 2 and the patient was discharged the same day. Histopathology revealed a rhabdomyosarcoma in form of a somatic transformation of the teratoma after chemotherapy with free margins of more than 2 cm. There were 4 negative lymph nodes in the en-bloc resected station 8, and 11 more in stations 6, 7, 9, 11 and 13. The case was discussed in the board for sarcoma. Since evidence for usefulness of adjuvant chemotherapy is lacking, no formal treatment was proposed besides follow-up.

Case 3
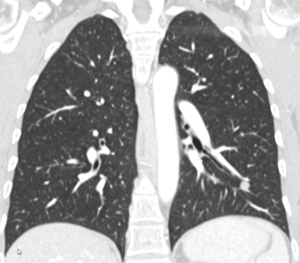
A 50-year-old female patient had undergone resection of a retroperitoneal leiomyosarcoma 3 years earlier. Twelve months ago retroperitoneal recurrence of the leiomyosarcoma was treated with neoadjuvant radiotherapy followed by en-bloc resection of the tumor, the right kidney, adrenal gland and gallbladder. The current follow up CT-scan showed a solitary 14 mm round lesion in the laterobasal segment (S9) of the left lower lobe, consistent with a lung metastasis, and no other signs for recurrence (Figure 5). The board for sarcoma proposed a metastasectomy. The FEV1 was 109% and the patient was fit for surgery. Due to the central location of the nodule we performed a uniportal S9-segmentectomy (Figure 6). The chest tube was removed on POD 1 and the patient was discharged home on POD 2. Histopathology confirmed a metastasis of the leiomyosarcoma with free resection margins. There were 9 negative lymph nodes (Stations 6, 7, 9, 10, 11 and 12). The board for sarcoma recommended no adjuvant treatment and to pursue the CT-scan follow up.

Tips and tricks
- Use of a soft wound protector minimizes spilling of the camera.
- Intercostal, subpleural nerve blockage with long lasting local anesthetic provides good pain control for the first 24 h (Figure 7).
 Figure 7 Intercostal, subpleural nerve blockage with long lasting anesthetic (18). Available online: http://www.asvide.com/article/view/30682
Figure 7 Intercostal, subpleural nerve blockage with long lasting anesthetic (18). Available online: http://www.asvide.com/article/view/30682 - Camera with 30° optic is held by the 1st assistant.
- Gentle constant aspiration on the operated lung can help deflate the lung (especially in case of relevant air trapping or of use of a bronchial blocker).
- Distal preparation of the vessels (arteries and veins) far into the parenchyma is mandatory for reliable anatomic identification.
- Thorough lymphadenectomy up to segmental station 13 helps identify the sublobar structures.
- Polymer locking ligation system (Hem-o-lok) combined with energy devices allows more flexibility for section of small vessels.
- Near infrared imaging with systemic injection of indocyanine green can be used:
- to confirm correctness of segmental artery (Figure 6).
- to identify the intersegmental plan (especially in mono-segmentectomy of the basilar pyramid) (Figures 2,6
). - can be repeated safely.
- An accessory port for stapling is useful in some rare cases.
Conclusions
The technique of uniportal segmentectomy is a good and safe, minimally invasive as well as lung-sparing technique for the resection of centrally located metastasis. In many of these cases also a less demanding surgical resection in form of a ‘wide-wedge’ would be possible, but at the prize of more resected lung parenchyma as well as a reduced control of resection margins. Finally uniportal surgery probably provokes less pleural adherences leaving options of redo-surgery for metachronous metastasis open.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Michel Gonzalez) for the series “Advancement in the Surgical Treatment of Pulmonary Metastasis” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2019.03.10). The series “Advancement in the Surgical Treatment of Pulmonary Metastasis” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this study and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Van Raemdonck D, Friedel G. The European Society of Thoracic Surgeons lung metastasectomy project. J Thorac Oncol 2010;5:S127-9. [Crossref] [PubMed]
- Internullo E, Cassivi SD, Van Raemdonck D, et al. on behalf of the ESTS pulmonary metastasectomy working group. Pulmonary metastasectomy: a survey of current practice amongst members of the European Society of Thoracic Surgeons. J Thorac Oncol 2008;3:1257-66. [Crossref] [PubMed]
- Perentes JY, Zellweger M, Gonzalez M. Personalized surgery for the management of pulmonary metastasis. J Thorac Dis 2018;10:52-5. [Crossref] [PubMed]
- Perentes JY, Krueger T, Lovis A, et al. Thoracoscopic resection of pulmonary metastasis: Current practice and results. Crit Rev Oncol Hematol 2015;95:105-13. [Crossref] [PubMed]
- Ripley RT, Downey RJ. Pulmonary metastasectomy. J Surg Oncol 2014;109:42-6. [Crossref] [PubMed]
- Zellweger M, Abdelnour-Berchtold E, Krueger T, et al. Surgical treatment of pulmonary metastasis in colorectal cancer patients: Current practice and results. Crit Rev Oncol 2018;127:105-16. [Crossref] [PubMed]
- Casiraghi M, De Pas T, Maisonneuve P, et al. A 10-year single-center experience on 708 lung metastasectomies: the evidence of the “international registry of lung metastases”. J Thorac Oncol 2011;6:1373-8. [Crossref] [PubMed]
- Gossot D, Radu C, Girard P, et al. Resection of pulmonary metastases from sarcoma: can some patients benefit from a less invasive approach? Ann Thorac Surg 2009;87:238-44. [Crossref] [PubMed]
- Greenwood A, West D. Is a thoracotomy rather than thoracoscopic resection associated with improved survival after pulmonary metastasectomy? Int Cardiovasc Thorac Surg 2013;17:720-4. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, McCarty TP, et al. A prospective study to determine the incidence of non-imaged malignant pulmonary nodules in patients who undergo metastasectomy by thoracotomy with lung palpation. Ann Thorac Surg 2011;91:1696-701. [Crossref] [PubMed]
- Mineo TC, Sellitri F, Fabbi E, et al. Uniportal non-intubated lung metastasectomy. J Vis Surg 2017;3:118. [Crossref] [PubMed]
- Lutz JA, Seguin-Givelet A, Gossot D. Thoracoscopic anterior segmentectomy of the right upper lobe (S3). J Vis Surg 2018;4:183. [Crossref]
- Gossot D, Lutz JA, Grigoroiu M, et al. Unplanned procedures during thoracoscopic segmentectomies. Ann Thorac Surg 2017;104:1710-7. [Crossref] [PubMed]
- Berry MF. Role of segmentectomy for pulmonary metastases. Ann Cardiothorac Surg 2014;3:176-82. [PubMed]
- Lutz JA, Kocher GJ. Uniportal left anterior segmentectomy (S3) with near infrared imaging (ICG) for better identification of intersegmental plane. Asvide 2019;6:076. Available online: http://www.asvide.com/article/view/30678
- Lutz JA, Kocher GJ. Uniportal left posterobasal segmentectomy (S10) with “en-bloc” lymphadenectomy (8+9) and accessory port for better stapling angle. Asvide 2019;6:077. Available online: http://www.asvide.com/article/view/30679
- Lutz JA, Kocher GJ. Uniportal left laterobasal segmentectomy (S9) with near infrared imaging (ICG) for confirmation of correctness of segmental artery and identification of intersegmental plane. Asvide 2019;6:078. Available online: http://www.asvide.com/article/view/30680
- Lutz JA, Kocher GJ. Intercostal, subpleural nerve blockage with long lasting anesthetic. Asvide 2019;6:079. Available online: http://www.asvide.com/article/view/30682
Cite this article as: Lutz JA, Kocher GJ. Uniportal segmentectomy: an alternative for resection of deeply situated lung metastases. J Vis Surg 2019;5:32.