
Custom-made elbow joint reconstruction with free osteoperiosteal fibula and iliac crest flaps after tumor resection: a case report
Introduction
Reconstruction of large bone defects around the elbow joint is a challenging issue in orthopedics, especially when performed in pediatric age. In recent years, many strategies have been proposed to restore bony defects following tumors resection (1). The non-microsurgical options are limited to allograft, prosthesis, or composite of both [allograft prosthesis composites (APC)]. All these alternatives present some disadvantages in the pediatric age: a frozen allograft of proper size is difficult to obtain and is prone to articular fracture and degenerative changes at medium term during internal repair (2). Modular megaprosthesis of distal humerus is indicated in adults, but prosthesis endurance is unpredictable, their size is not suitable for children, and they cannot provide growth. Custom made hemiarthroplasty of the distal humerus is another option, with concerns regarding elbow stability. Moreover, in case of subtotal humerus resection, prosthetic anchorage into the small residual proximal humerus would not be possible and prosthetic reconstruction would require a total humerus custom made lengthening hemiarthroplasty (3,4). Autologous reconstruction of massive bone losses of the distal humeral epiphysis have been described in the literature, but published case numbers are small with heterogeneous diagnosis (5-7). Since the proximal growth plate is responsible for 80% of humeral bone growth (8), there is no need to transfer a vascularized proximal fibular epiphysis, as required in distal radius reconstruction (9-12). The purpose of this article is to introduce a new single-step technique to reconstruct humeral epiphysis and diaphysis following Ewing’s sarcoma resection using a vascularized transfer of iliac crest and fibula grafts reinforced with an allograft.
Methods
Patient
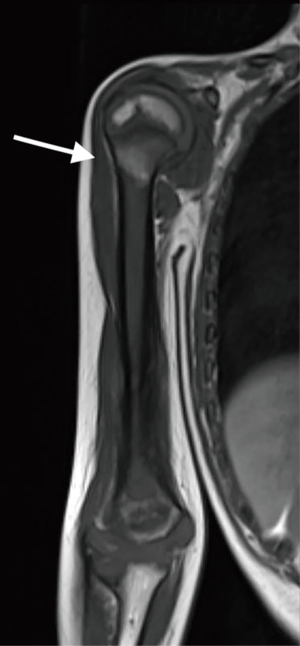
A 5-year-old patient presented with a lytic lesion in the right distal humerus. Magnetic resonance imaging (MRI) was suggestive of malignant bone tumor with extension to the surrounding soft tissue (Figure 1). No metastatic lesions were found on chest CT scan and bone scintigraphy. An open biopsy was performed and demonstrated Ewing’s sarcoma. One month after completion of neoadjuvant chemotherapy, he underwent resection of humeral diaphysis and distal epiphysis, and immediate reconstruction with free iliac crest and fibula flaps. An allograft was used to reinforce the vascularized transplants in the diaphyseal part.
Surgical technique
The operation was performed using a two-team approach in conjunction with orthopedic surgeons performing the resection of the tumor and osteoarticular reconstruction.
Patient was placed in supine position. A double curved-S-incision was used, starting proximally at coracoids process into a deltopectoral approach and prolonged on the lateral aspect of the arm and elbow. Insertions of deltoid, pectoralis major, latissimus dorsi and teres major were cut from the humerus. A careful blunt dissection was performed to separate and protect the neurovascular bundle (median nerve, and the brachial artery and vein); radial nerve in the arm and ulnar nerve in the epitrochlear fossa were identified and preserved. Biceps was preserved while part of the brachialis, brachioradialis and triceps muscle were resected and left for tumor coverage. Elbow arthrotomy sparing medial and lateral ligaments was performed and the proximal humerus was cut with oscillating saw 1.5 cm below the proximal growth plate. Frozen section of the residual medullary canal ensured negative margins (Figure 2).

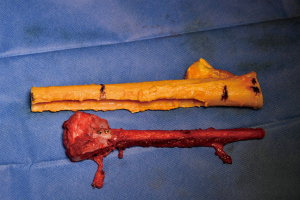
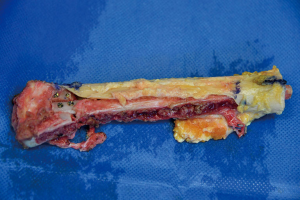
Simultaneously, we harvested the contralateral vascularized bony flaps (Figures 3,4). A 4 cm × 3 cm iliac crest graft was shaped to recreate the humeral epiphysis and fixed to a 13 cm long fibula flap with two 2.4 mm screws, then the two pedicles were anastomosed in end-to-end fashion. The cortical allograft was cut to match the resection defect and its medullary canal was enlarged to allow fibula concentric assembling (Figure 5). The fibular flap was then passed through the intramedullary canal of the allograft, and the iliac crest was fixed to the distal part of the allograft with two 2.4 mm screws (Figure 6). The construct was inserted in the recipient site and, under fluoroscopic control, fixed with the proximal part of the humerus with a 2.7 mm T-plate and screws (Figure 7). Due to the small size of the residual proximal humerus, the osteosynthesis was performed across the growth plate with the insertion of two epiphyseal screws (Figure 8). Medial and lateral collateral ligaments of the elbow were fixed on the iliac bone with suture anchors and the deep brachial artery with its comitant vein were used as donor vessels for an end-to-end anastomosis. The radial nerve was transposed anteriorly to avoid nerve compression (Figure 9).


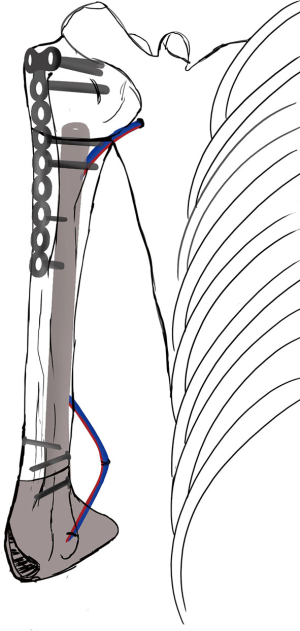

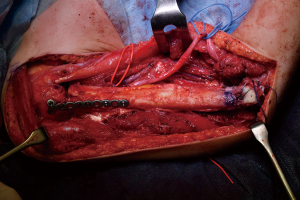
The elbow-joint was stabilized with a temporary trans-olecranon Kirschner wire, muscles tendons were reinserted, while pectoralis major was sutured onto the deltoid and brachioradialis onto triceps muscle. Under fluoroscopic control, tibiofibular metaphyseal synostosis at the donor-site ankle was performed with a 2.7 mm screw. Surgical sites were closed primarily and one suction drain was placed in each of them.
Post-operative management and outcome
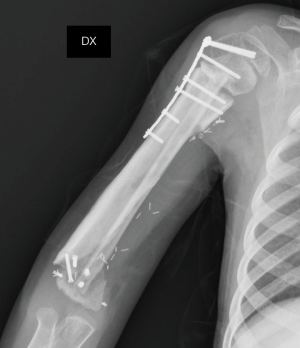
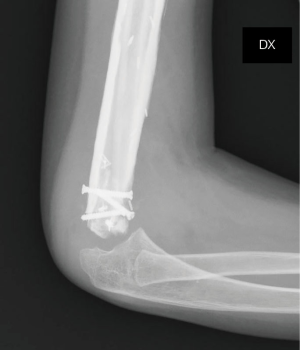
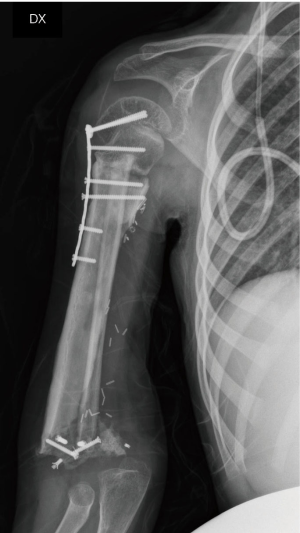
On the postoperative radiograph the construct was well-positioned and stable. The arm was immediately immobilized in Desault bandage. One month after surgery, the trans-olecranon Kirschner wire was removed and shoulder and arm brace was positioned for other 4 weeks, encouraging daily progressive and careful elbow passive mobilization. Active mobilization training of the shoulder and elbow was started at 8 weeks (Figures 10,11). Postoperative chemotherapy was delivered as soon as the wound was healed according to National protocol. There were no deep infections at either the donor or recipient sites. After 6 months, the patient fell on the ground reporting a traumatic minimally displaced fracture of the distal iliac crest flap without hardware loosening. No revision surgery was performed. After 8 months follow up, bony union was documented radiographically with no signs of local recurrence. No signs of metastasis were detected from the chest CT scan as well. At latest follow-up at 10 months, imaging confirmed allograft and flap union at proximal osteotomy and vascularized fibula diaphyseal hypertrophy (Figure 12). At clinical examination, the patient presented a full range of motion of the shoulder. The elbow was stable, with flexion from 45° to 110° and full pronosupination.
Equipment preference card
- Cortical 2.4 mm screws have been used for fixation of the fibula flap to the iliac crest flap;
- 2.7 mm plate with cortical screws have been used to fix the flaps to proximal humerus;
- Kirschner wires are useful for temporary fixation and to be used as a guide, under fluoroscopy, for proximal osteotomy;
- High speed burr can be used to shape the allograft to the adequate size.
Tips, tricks and pitfalls
- The proximal juxta-articular osteotomy can be performed using Kirschner wires to guide the oscillating saw under fluoroscopy;
- In growing children, valgus deformity of the donor site ankle can be expected as a complication of vascularized fibula harvest. In this case, preventive tibiofibular screw fixation was performed. This is advisable in children if the residual fibula is less than 6 cm in length (17);
- The massive allograft must be molded using oscillating saw and high-speed burr, performing a hemidiaphysectomy in the distal part of the humerus, to avoid impeachment with the vascular pedicle of the flaps. In the proximal part, the diaphysis can be maintained intact, to preserve mechanical strength, reaming the medullary canal to be large enough to accept the vascularized fibula in a concentric assembling;
- During bony flaps harvesting, the use of piezoelectric surgery provides safely and precise cutting of the bone;
- As soon as the proximal osteotomy is healed and solid union is radiographically evident, proximal epiphyseal screws should be removed and replaced with metaphyseal screws with distal orientation in order to free the growth plate and avoid epiphysiodesis.
Conclusions
In conclusion, a satisfactory humeral and elbow-joint reconstruction can be obtained with this technique in repairing an osteoarticular massive defect following oncological resections. The alternative to this procedure would have been a total humerus custom made lengthening hemiarthroplasty. When it is not contraindicated, autologous biologic reconstruction is preferable to endoprosthesis, due to their high risk of complications and surgical revisions at long term. Particularly, the iliac bone provides the possibility to adequately shape the graft to fit the distal humeral part of the elbow-joint into the olecranon while using the fibula to restore the diaphysis. The inclusion of an allograft is useful to protect the vascularized fibular graft during the hypertrophy process, reducing complication rates. Long-term outcomes of this articular reconstruction still remain unknown: additional studies will be required to determine the long-term success, especially in young patients.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2019.03.08). MI serves as an unpaid editorial board member of Journal of Visualized Surgery from Oct 2018 to Sep 2020. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the parents of the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Houdek MT, Wagner ER, Wyles CC, et al. New options for vascularized bone reconstruction in the upper extremity. Semin Plast Surg 2015;29:20-9. [Crossref] [PubMed]
- Ogink PT, Teunissen FR, Massier JR, et al. Allograft reconstruction of the humerus: Complications and revision surgery. J Surg Oncol 2019;119:329-35. [Crossref] [PubMed]
- Henrichs MP, Liem D, Gosheger G, et al. Megaprosthetic replacement of the distal humerus: still a challenge in limb salvage. J Shoulder Elbow Surg 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Capanna R, Muratori F, Campo FR, et al. Modular megaprosthesis reconstruction for oncological and non-oncological resection of the elbow joint. Injury 2016;47:S78-83. [Crossref] [PubMed]
- Cavadas PC, Landin L, Thione A, et al. Reconstruction of massive bone losses of the elbow with vascularized bone transfers. Plast Reconstr Surg 2010;126:964-72. [Crossref] [PubMed]
- Hattori Y, Doi K, Pagsaligan JM, et al. Arthroplasty of the elbow joint using vascularized iliac bone graft for reconstruction of massive bone defect of the distal humerus. J Reconstr Microsurg 2005;21:287-91. [Crossref] [PubMed]
- Scaglioni MF, Chang EI, Gur E, et al. The role of the fibula head flap for joint reconstruction after osteoarticular resections. J Plast Reconstr Aesthet Surg 2014;67:617-23. [Crossref] [PubMed]
- Pritchett JW. Growth plate activity in the upper extremity. Clin Orthop Relat Res 1991;235-42. [PubMed]
- Innocenti M, Delcroix L, Balatri A. Vascularized growth plate transfer for distal radius reconstruction. Semin Plast Surg 2008;22:186-94. [Crossref] [PubMed]
- Innocenti M, Delcroix L, Manfrini M, et al. Vascularized proximal fibular epiphyseal transfer for distal radial reconstruction. J Bone Joint Surg Am 2005;87:237-46. [Crossref] [PubMed]
- Innocenti M, Ceruso M, Manfrini M, et al. Free vascularized growth-plate transfer after bone tumor resection in children. J Reconstr Microsurg 1998;14:137-43. [Crossref] [PubMed]
- Innocenti M, Baldrighi C, Menichini G. Long Term Results of Epiphyseal Transplant in Distal Radius Reconstruction in Children. Handchir Mikrochir Plast Chir 2015;47:83-9. [Crossref] [PubMed]
- Innocenti M, Lucattelli E, Brogi M, et al. Resection of an Ewing’s sarcoma of the right distal humerus. Asvide 2019;6:065. Available online: http://www.asvide.com/article/view/30501
- Innocenti M, Lucattelli E, Brogi M, et al. Harvesting technique of a fibula free flap. Asvide 2019;6:066. Available online: http://www.asvide.com/article/view/30502
- Innocenti M, Lucattelli E, Brogi M, et al. Harvesting technique of an iliac crest free flap. Asvide 2019;6:067. Available online: http://www.asvide.com/article/view/30503
- Innocenti M, Lucattelli E, Brogi M, et al. Assembly of the bony flaps with the allograft, osteosynthesis with the humeral head, reconstruction of medial and lateral collateral ligaments of the elbow. Asvide 2019;6:068. Available online: http://www.asvide.com/article/view/30504
- Omokawa S, Tamai S, Takakura Y, et al. A long-term study of the donor-site ankle after vascularized fibula grafts in children. Microsurgery 1996;17:162-6. [Crossref] [PubMed]
Cite this article as: Innocenti M, Lucattelli E, Brogi M, Totti F, Campanacci DA. Custom-made elbow joint reconstruction with free osteoperiosteal fibula and iliac crest flaps after tumor resection: a case report. J Vis Surg 2019;5:28.


