Primary spontaneous pneumothorax (PSP): the role of VATS and a new paradigm
The two main objectives in the therapeutic management of patients with PSP are: restoring negative pressure of the pleural space and reducing the possibility of recurrence.
The strategy of non-invasive treatment
The strategy of non-invasive, conservative treatment with observation of evolution, will be reserved only for asymptomatic patients and with small volume pneumothorax, less than 15% (apex/dome distance <3 cm). Considering that the communication between the alveolus and the pleural space has been eliminated, in a prediction of 1.25% volume per day of reabsorption, at the end of twelve days the pneumothorax will be totally absorbed. If the patient is hospitalized, the use of supplemental oxygen may accelerate this reabsorption.
However recurrence rates, may reach 50% and approximately 40% of these patients will require pleural drainage. Although it is a non-invasive treatment, it is not simple to monitor the evolution of the pneumothorax camera, requiring sequential radiological examinations and with no preventive action (1).
Percutaneous aspiration X tubular pleural drainage
For patients with greater volume pneumothorax, above 15–20%, it will be indicated the removal of accumulated air through drainage. This procedure can be done by pleural puncture with percutaneous aspiration or by the insertion of a thoracic drain.
The percutaneous aspiration is a less invasive and less costly treatment indicated to pneumothorax with volumes up to 15%. Similar to conservative treatment.
Aspiration does not prevent relapse in 25% of cases. When compared to tubular pleural drainage, the successful rate for complete air drainage is lower, 67% and 93%, respectively (2).
Thus, isolated pleural drainage is able to prevent recurrence of pneumothorax, in approximately 35%, similar to methods that do not add preventive procedures, such as pleurodesis or resection of blebs (3-5).
Surgical treatment in first episode
Even the recent technological advances and the growing number of publications indicating the need for review the definitive surgical approach to PSP were not able to change the classic guideline. However, they have undoubtedly brought into question the need to add new items to the list.
The increase availability of minimally invasive surgery to all surgeons is established as a method of choice in surgical treatment of these patients.
The reduction in hospitalization time, impact on care costs, less postoperative pain, high therapeutic resolution by the extinction of the cause and prevention of relapse, reduction of the emotional impact considering the chances of recurrence around 30%, after the first episode and the possibility of early return to the usual activities mainly to young population.
These variables support the reflection on method indication at the first episode of PSP. Why not? (6-11).
The first manifestations of this indication occurred in trials from the late 1990s to the early 2000s (12-14).
A panel of experts who sought consensus, about the management of PSP, 15% of them considered the discussion with the patient, to decide the timing of the intervention, in the first episode, since through video-assisted thoracoscopy (15).
Even without the use of video-surgery, the indication of treatment through axillary thoracotomy and abrasive pleurodesis in PSP first episode was effective. In a series of 23 patients who underwent to a surgery in the first episode, there was no recurrence in three years of follow-up, reduced length of pleural drainage (2 days) and hospital stay (5 days). Concluding this study, the authors consider the possibility of surgical approach in the first episode of the PSP (16).
The studies that compared the strategies for the first episode, pleural drainage vs. video surgery, are eloquent in showing the advantages of second one when we analyze the pleural drainage duration, hospitalization, care costs and patient satisfaction (17).
A study including 70 patients prospectively admitted with PSP and randomized to treatment with pleural drainage (group 1) vs. video-surgery with resection of blisters/blebs and apical pleurectomy (group 2), the main outcomes were prolonged airflow (>6 days), that occurred in 11% vs. 5.7%; recurrence 28% vs. 2.8%; mean hospital time 9–12 vs. 4–6 days; mean care costs (hospitalization and material) $2,750.00 per patient vs. $1,925.00. In this study, the indication of definitive surgical treatment in the first episode was justified by the patients and hospital managers interests, showing that the costs of relapses in group 1, who subsequently adhered to surgery, could have been avoided if they underwent surgical treatment as the first choice (18).
The generalization of the indication for surgical intervention in the first episode, even if stimulated by the excellent results mentioned, should be viewed with some caution, without losing the enthusiasm and the prospect of being able to offer the most effective therapy for the control of the disease. The need to select, within the population of patients with PSP, those with a higher risk of recurrence is central to the change in this therapeutic paradigm, still supported by the most recent guidelines. There is currently no clinical evidence or diagnostic test that is proven to be effective and capable of identifying patients at risk of relapse (15,19,20).
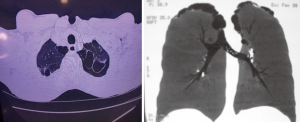
Considering the consensus that bubble/bleb rupture is present in the etiology of the disease in the vast majority of cases of PSP (Figure 1), it is reasonable to imagine that in the analysis of this circumstance, patients with a higher probability of recurrence may be identified. In this sense, imaging studies, fundamentally high resolution computed tomography, impose themselves in the search for anatomical criteria capable of predicting the greater or lesser risk of recurrence and, in identifying them, propose that definitive treatment can be instituted already in the first episode, with undeniable benefit to the patient and to the assistance models as previously mentioned.
A recent study, led by Casali et al. (21), evaluated retrospectively 176 patients who had first episode of PSP and received conservative treatment: pleural drainage or resting and observation. It aimed at identifying the correlation between ipsilateral or contralateral recurrence and the presence of blebs or blisters detected through high resolution tomography. By creating a severity score based on the bullous-type changes (I) Blebs or blisters, (II) number (single or multiple) and (III) distribution (unilateral or bilateral) identifies in these patients, they were able to correlate the severity to the relapse rates. In patients who presented blebs/blisters (110/176) at the ipsilateral recurrence rate was 68.1% (75/110 patients), which depicted the positive predictive value of tomographic findings. When multiple lesions were detected, relapse rate increased to 82%. On the other hand, in patients without bleb/blister tomography findings, the recurrence rate was 6.1%, with a negative predictive value of 93.9%. The risk of contralateral recurrence was 19% in patients with positive radiological findings and absent in those whit no contralateral alterations. In a multivariate analysis that included sex, age, body mass, smoking and pneumothorax, only the presence of blebs/blisters was significant for the risk of relapse (P<0.001) and 18 as odds ratio.
Other similar studies established a correlation between tomographic findings and recurrence, however without the same design, follow-up and number of patients (22-24).
These studies allow us to support the higher probability of relapse according to the findings of emphysema like alterations in high resolution computed tomography and to argue the indication of early intervention and recurrence prevention. Likewise they support the more conservative behavior in patients without evidence of these findings.
There is no doubt that other studies will be necessary and it gives certainty that the current indications for surgical treatment of primary spontaneous pneumothorax should be questioned.
Contra-lateral prophylactic surgical treatment
Whereas the excellent results of video surgery for PSP treatment, the increasing number of studies proposing surgical treatment for the first uncomplicated ipsilateral episode in the presence of emphysema-like radiological alterations, as previously mentioned, many authors are presenting cases that denotes definitive preventive surgical treatment for patients in the first episode when the high resolution tomography showed contralateral disease. The first works that introduced this therapeutic premise were based on the rates of contralateral recurrence with the presence of tomographic changes. Sihoe et al. identified 26.7% of contralateral pneumothorax in 15 patients with tomographic evidence of blebs or blisters from a small series of 28 successive patients with PSP. They conclude that chest CT can be useful to identify group of patients and indicate contralateral preventive surgical treatment in specific ones (25).
The bilateral and simultaneous video-assisted intervention does not add morbidity to the procedure, keeping satisfactory results in controlling the disease (26,27).
A late prospective study attempted to answer whether there is indication for contralateral prophylactic treatment in patients with PSP.
A number of 86 patients with PSP (53.5% with bilateral disease) sharing the same demographic variables (gender, age, smoking and body mass) were divided into three groups. Group 1 (N=35) with unilateral disease, were submitted to ipsilateral video-surgery; Group 2 (N=35) bilateral disease, underwent unilateral and ipsilateral video surgery; Group 3 (N=16) bilateral disease, were submitted to bilateral video surgery at the same anesthetic time. The rates of contralateral recurrence in Groups 1, 2 and 3 were 2.8%, 17.14% and none, respectively. Except for prolonged air leakage in 7 cases, no other morbidity was identified, no recurrence on the operated side, and overall satisfaction with the aesthetic result. The surgical time was longer (P<0.001) in patients submitted to simultaneous bilateral treatment. Even with a small number of patients, this study was able to demonstrate that simultaneous bilateral preventive approach did not add morbidity, relieved the anxiety of relapse risk. Prior discussion with patients and family members about informed consent for the procedure was very valued. Considering that this strategy also avoids a second hospitalization, anesthesia and another surgery the answer was yes, prophylactic treatment should be indicated for these patients (28).
Thus, definitive bilateral prophylactic surgical treatment aims to be a safe therapeutic option for patients with PSP, in the presence of bilateral measurable disease by chest computed tomography. Obviously, it will be necessary more large studies to support an unquestionable indication, to evaluate the strategy costs and to answer why a great part of patients with potential risk of relapse will never have it. However, excluding it from the therapeutic option, especially from the discussion about morbidity and relapse with the patient and his relatives, is to restrict the access to a safe method with very satisfactory results for a young and healthy population of patients, who is afraid of experiencing sudden pain, new drainage and its inherent pain, return to the hospital and activity restriction again.
Operative technique
There is no doubt that minimally invasive surgery overcomes other alternatives for the surgical treatment of primary spontaneous pneumothorax. Conventional thoracotomies, axillary thoracotomies and sternotomy are reserved for very specific cases. The effort to offer methods that combine minimal morbidity, effective therapeutic resolution and rapid return to regular activities is consistent as a therapeutic rule and achieves a larger dimension if indicated for a benign disease affecting a young and healthy population.
Most patients undergo general anesthesia, a double lumen tube to promote selective ventilation. Instrumental entrance portals are defined according to the surgeon’s choice and familiarity, ranging from uniportal access to three or four toracoportes. The possibility of surgery with the non-intubated patient is a reality in contemporary thoracic surgery (29-31).
When indicated surgery with non-intubated patients for the PSP, it showed the same technique effectiveness of the technique with general anesthesia, which can present a reduction in hospital time (31).
Other access routes such as subxiphoid or transareolar approach seek alternatives that further reduce pain and improve aesthetic effects (32,33).
The following technical routines used for bilateral approach of the Department of Thoracic Surgery of General Hospital-Foundation University of Caxias do Sul:
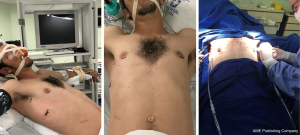
- Patient in dorsal decubitus with elevation of 30–45° (Figure 2).
- Abducted arms for armpit exposure (Figure 2).
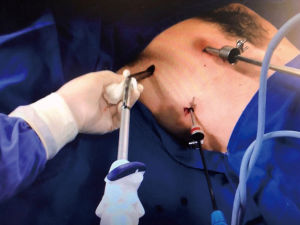
- Two toracoportes for patients without previous drainage and three toracoportes to take advantage of the previous drainage incision (Figure 3).
- In men, 30° optic access is subareolar and in females in the submammary sulcus.
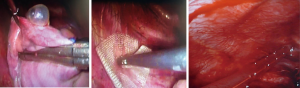
- A small 2 cm working incision in the axillary void allows the passage of the stapler and also of the instruments for performing abrasive pleurodesis or apical pleurectomy (Figures 3,4
). - If you already have drainage, the incision can be used to introduce the pleural drainage.
- Otherwise, the drain can be placed in the axillary incision in men or submammary in women.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Ricardo M. Terra and Paula A. Ugalde) for the series “Minimally Invasive Surgery - Robotics and VATS in Brazil” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2019.02.04). The series “Minimally Invasive Surgery - Robotics and VATS in Brazil” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Seremetis MG. The management of spontaneous pneumothorax. Chest 1970;57:65-8. [Crossref] [PubMed]
- Andrivet P, Djedaine K, Teboul JL. Spontaneous pneumothorax: comparison of thoracic drainage vs. immediate or delayed needle aspiration. Chest 1995;108:335-9. [Crossref] [PubMed]
- Schoenenberger RA, Haefeli WE, Weiss P, et al. Timimg of invasive procedures on therapy for primary and secondary spontaneous pneumothorax. Arch Surg 1991;126:764-66. [Crossref] [PubMed]
- Campos JR, Werebe EC, Vargas FS, et al. Respiratory failure due to insufflated talc. Lancet 1997;349:251-2. [Crossref] [PubMed]
- Maskell NA, Lee YC, Gleeson FV, et al. Randomized trials describing lung inflammation after pleurodesis with talc of varying particle size. Am J Respir Crit Care Med 2004;170:377-82. [Crossref] [PubMed]
- Morimoto T, Fukui T, Koyama H, et al. Optimal strategy for the first episode of primary spontaneous pneumothorax in Young men. J Gen Intern Med 2002;17:193-202. [Crossref] [PubMed]
- Naunheim KS, Mack MJ, Hazelrigg SR, et al. Safety and efficacy of video-assisted thoracic surgical techniques for the treatment of spontaneous pneumothorax. J Thorac Cardiovasc Surg 1995;109:1198-203. [Crossref] [PubMed]
- Treasure T. Minimally invasive surgery for pneumothorax: the evidence, changing practice and current opinion. J R Soc Med 2007;100:419-22. [Crossref] [PubMed]
- Barker A, Maratos EC, Edmonds L, et al. Recurrence rates of video-assisted thoracoscopic versus open surgery in the prevention of recurrent pneumothoraces: a systematic review of randomized and non-randomized trials. Lancet 2007;370:329-35. [Crossref] [PubMed]
- Treasure T. Minimal access surgery for pneumothorax. Lancet 2007;370:294-5. [Crossref] [PubMed]
- Vohra HA, Adamson L, Weeden DF. Does video-assisted thoracoscopic pleurectomy result in better outcomes than open pleurectomy for primary spontaneous pneumothorax? Interact Cardiovasc Thorac Surg 2008;7:673-7. [Crossref] [PubMed]
- Waller DA, Forty J, Morritt GN. Video-assisted thoracoscopic surgery versus thoracotomy for spontaneous pneumothorax. Ann Thorac Surg 1994;58:372-6. [Crossref] [PubMed]
- Massard G, Thomas P, Wihlm JM. Minimally invasive management for first and recurrent pneumothorax Ann Thorac Surg 1998;66:592-9. [Crossref] [PubMed]
- Cole FH, Khandekar A, Maxwell J, et al. Video-assisted thoracic surgery: primary treatment for spontaneous pneumothorax. Ann Thorac Surg 1995;60:931-3; discussion 934-5. [Crossref] [PubMed]
- Baumann MH, Strange C, Heffner JE, et al. Management of spontaneous pneumothorax. An American College of Chest Physicians. Delphi Consensus Statement. Chest 2001;119:590-602. [Crossref] [PubMed]
- Pinto D, Leite AG, Pelin FD, et al. Surgical treatment of primary spontaneous pneumothorax in the first episode. Jornal de Pneumologia 2001;27:153-7.
- Sawada S, Watanabe Y, Moriyama S. Video-assisted thoracoscopy surgery for primary spontaneous pneumothorax. evaluation of indications and long-term outcome compared with conservative treatment and open thoracotomy. Chest 2005;127:2226-30. [Crossref] [PubMed]
- Torresini G, Vicarli M, Divisi D, et al. Is video-assisted thoracic surgery justified for first spontaneous pneumothorax? Eur J Cardiothorac Surg 2001;20:42-5. [Crossref] [PubMed]
- MacDuff A, Arnold A, Harvey JBTS Pleural Disease Guideline Group. Management of spontaneous pneumothorax: British Thoracic Society pleural disease guideline 2010. Thorax 2010;65:ii18-31. [Crossref] [PubMed]
- De Leyn P, Lismonde M, Ninane V, et al. Guidelines Belgian Society of Pneumology. Guidelines on the management of spontaneous pneumothorax. Acta Chir Belg 2005;105:265-7. [Crossref] [PubMed]
- Casali C, Stefani A, Ligabue G, et al. Role of Blebs and Bullae Detected by High-Resolution Computed Tomography and Recurrent Spontaneous Pneumothorax. Ann Thorac Surg 2013;95:249-55. [Crossref] [PubMed]
- Huang TW, Lee SC, Cheng YL, et al. Contralateral recurrence of primary spontaneous pneumothorax. Chest 2007;132:1146-50. [Crossref] [PubMed]
- Mitlehner W, Friedrich M, Dissmann W. Value of computer tomography in the detection of bullae and blebs in patients with primary spontaneous pneumothorax. Respiration 1992;59:221-7. [Crossref] [PubMed]
- Martínez-Ramos D, Angel-Yepes V, Escrig-Sos J, et al. Usefulness of computed tomography in determining risk of recurrence after a first episode of primary spontaneous pneumothorax: therapeutic implications. Arch Broncopneumol 2007;43:304-8. [Crossref] [PubMed]
- Sihoe AD, Yim AP, Lee TW, et al. Can CT scanning be used to select patients with unilateral primary spontaneous pneumothorax for bilateral surgery? Chest 2000;118:380-3. [Crossref] [PubMed]
- Lang-Lazdunski L, de Kerangal X, Pons F, et al. Primary Spontaneous Pneumothorax: One-Stage Treatment by Bilateral Videothoracoscopy. Ann Thorac Surg 2000;70:412-7. [Crossref] [PubMed]
- Noh D, Keum DY, Park CK. Outcomes of Contralateral Bullae in Primary Spontaneous Pneumothorax. Korean J Thorac Cardiovasc Surg 2015;48:393-7. [Crossref] [PubMed]
- Chou SH, Li HP, Lee JY, et al. Is prophylactic treatment of contralateral blebs in patients with primary spontaneous pneumothorax indicated? J Thorac Cardiovasc Surg 2010;139:1241-5. [Crossref] [PubMed]
- Nezu K, Kushibe K, Tojo T, et al. Thoracoscopic wedge resection of blebs under local anesthesia with sedation for treatment of spontaneous pneumothorax. Chest 1997;111:230-5. [Crossref] [PubMed]
- Pompeo E, Mineo D, Rogliani P, et al. Feasibility and results of awake thoracoscopic resection of solitary pulmonary nodules. Ann Thorac Surg 2004;78:1761-8. [Crossref] [PubMed]
- Pompeo E, Tacconi F, Mineo D, et al. The role of awake video-assisted thoracoscopic surgery in spontaneous pneumothorax. J Thorac Cardiovasc Surg 2007;133:786-90. [Crossref] [PubMed]
- Liu CY, Lin CS, Liu CC. Subxiphoid single-incision thoracoscopic surgery for bilateral primary spontaneous pneumothorax. Wideochir Inne Tech Maloinwazyjne 2015;10:125-8. [Crossref] [PubMed]
- Lin JB, Chen JF, Lai FC, et al. Transareolar pulmonary bullectomy for primary spontaneous pneumothorax. J Thorac Cardiovasc Surg 2016;152:999-1005. [Crossref] [PubMed]
Cite this article as: Filho DRP, Pinto BM, Pinto VM. Primary spontaneous pneumothorax (PSP): the role of VATS and a new paradigm. J Vis Surg 2019;5:27.