
Management of intraoperative bleeding during video-assisted thoracoscopic surgery
Introduction
Advances in thoracoscopic tools have led to an increase in thoracic procedures being performed by video-assisted thoracic surgery (VATS). In Japan, approximately 70% of lung cancer resections are performed by VATS (1). Therefore, thoracic surgeons should have the ability and experience to tackle intraoperative bleeding not only during open surgery but also during thoracoscopic surgery. From that standpoint, VATS is advantageous because the surgical procedure can be recorded, and thus a bleeding event can be reviewed, shared, and analyzed for further prevention and management.
Prevention of bleeding during VATS
An important aspect in the management of intraoperative bleeding is to prevent its occurrence and to be prepared in case it does occur. A preoperative workup should be routinely performed, and situations that could possibly lead to intraoperative bleeding should be predicted, such as extensive adhesion, calcified lymph nodes, or fused fissure. We should pay attention when a patient has a history of pneumonia, pleuritis, and we must carefully check the computed tomography images preoperatively. Basic rules for preventing bleeding include gentle handling of tissues, accurate recognition of anatomical structures, and appropriate use of energy devices. Energy devices have specific issues, such as thermal diffusion at the tip or side surface.
In terms of the surgical view, VATS has both advantages and disadvantages compared with open surgery (2). A magnified view of the surgical field facilitates recognition of detailed anatomy, with visualization from various directions that may be occasionally difficult to obtain during thoracotomy. Conversely, areas outside the monitored view are potentially dangerous blind sites, and excessive magnification or incompletely collapsed lung can lead to an insufficient view of the entire anatomy. We should pay attention to surgical tools when handling them outside of the monitored view.
To avoid misidentification or oversight of important vessels, a detailed observation of the anatomical vasculature both before and during surgery is beneficial. Furthermore, recognizing atypical vessels, such as aberrant V2 or V6, is required while dissecting the interlobar fissure. We recommend using three-dimensional analysis prior to surgery because it allows an intuitive recognition of the entire pulmonary bronchovascular anatomy that can be easily shared with the surgical team (2-5).
Preparation for bleeding during VATS
This step involves not only the chief operating surgeon but also the assistant surgeon, scopist, nurses, and anesthetist. A hospital infrastructure facilitating rapid blood transfusions in cases of unexpected bleeding would also be beneficial in institutions that routinely perform major lung resections.
When conversion from VATS to thoracotomy is necessary, an access site used for VATS will be elongated. Usually, the intercostal space used as access window will be further used for thoracotomy (6). Accordingly, the initial port placement adopted for VATS can affect the thoracotomy when conversion is required. Thus, whenever we perform a VATS procedure, we have to consider how we would convert from the VATS port placement to thoracotomy in a case of uncontrollable bleeding or if there is a need for discontinuation of the VATS approach. In our surgical team, the access window is mostly placed in the 4th or 5th intercostal space, which allows conversion to routine thoracotomy using the same intercostal space.
Classification and management of bleeding (Table 1)
Table 1
| Grade | 1 (oozing) | 2 (minor/mild) | 3 (major/moderate) | 4 (heavy/more serious) | Reference |
|---|---|---|---|---|---|
| Definition | Oozing from adhered lung or thoracic wall | Bleeding from vessels <3–4 mm | Significant bleeding from proximal PA, PV | ( |
|
| Small tears of distal PA | Bleeding from systemic circulation (left brachiocephalic vein, superior vena cava, arteries, etc.) | ||||
| Bruising of PA branch | Bleeding <500 mL | Bleeding >500 mL | |||
| Injury to vascular stumps | |||||
| Management | Compression | Compression | Compression (sponge stick, suction, lung parenchyma) | ( |
|
| Direct coagulation | Coagulation, use of energy device | Use of hemostats, sealants, or adhesives after initial compression | |||
| Hemostats | Hemostats, sealants | Clipping or suturing according to situation | |||
| Elongation of access port incision | Reapplication of stapler as a temporary clamp if caused by mechanical failure during stapling | ||||
| Early control is required to avoid development to grade 3/4 bleeding | Isolation of pulmonary artery and preparation for clamping | ||||
| Preparation for blood transfusion | |||||
| Conversion to thoracotomy | |||||
PA, pulmonary artery; PV, pulmonary vein; VATS, video-assisted thoracic surgery.
A classification that permits rapid assessment is required for prompt decision-making. Currently, the classification and grading of bleeding is diverse among surgeons and management is accordingly different. Demmy et al. classified bleeding events as “mild” and “more serious” in an articledescribing trouble management encountered during VATS lobectomies (6). Similarly, Gonzalez-Rivas classified bleeding into three types: “oozing,” “minor,” and “major” (7). Management of bleeding is affected by its volume and the site of bleeding. Oozing primarily refers to bleeding from adhered lung or thoracic wall. Minor or mild bleeding comprises bleeding from vessels smaller than 3–4 mm, bleeding from small tears of the distal pulmonary artery, bruising of pulmonary artery branches, and injuries to vascular stumps. Major or moderate bleeding refers to significant bleeding from proximal pulmonary arteries or veins. Finally, heavy or more serious bleeding would be defined as bleeding from pulmonary vessels exceeding 500 mL (7,8). In an attempt to rapidly assess and classify VATS bleeding in a generalized manner, we termed oozing as grade 1, minor/mild bleeding as grade 2, major/moderate bleeding as grade 3, and heavy/more serious bleeding as grade 4 (Table 1).
For grade 1 bleeding, compression, direct coagulation, or hemostatic material is recommended. Grade 2 bleeding should be controlled by the application of mild pressure. Additionally, thrombostatic material, biological sealant, coagulation, and energy devices can be used. Extension of access port incision should also be considered in grade 2 bleeding because early control is essential to avoid further progression to grade 3 or 4 bleeding. For grade 3 to 4 bleeding, the first and most important method is also direct compression. Manual compression is not possible during VATS; thus, compression may be applied via a sponge stick, suction, or the adjacent lung parenchyma. Options for hemostasis following initial compression include use of hemostats, sealants, vascular clips, or direct suturing. Because clips are prone to accidental displacement, the use of two clips and/or additional sealing of the proximal end are recommended. Direct suture should be performed if these previous methods fail or are difficult to perform. However, suturing of a critical bleeding site by VATS can be challenging. Suturing may be accompanied by isolation of the main pulmonary artery and preparation for clamping. If bleeding occurs during removal of a stapler, clamping of the bleeding site by reapplication of the stapler can be considered.
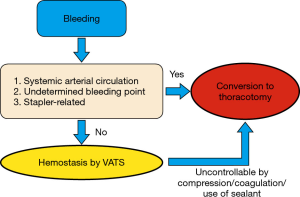
During conversion to thoracotomy, tamponade using a sponge stick or application of ring forceps should be performed. Bleeding events that require conversion to thoracotomy is a potential threat to patient safety. The surgical team needs to construct a common algorithm that they can rapidly refer to in case of uncontrollable bleeding. An effective algorithm should include the definition of the type of bleeding that requires conversion and a simple sequence of procedures. At our institute, we currently take an intraoperative decision based on a simple algorithm (Figure 1). We consider prompt conversion to thoracotomy when (I) the bleeding originates from a systemic arterial circulation; (II) the point of bleeding cannot be determined; or (III) when the bleeding is caused by stapler-related problems (i.e., stapler failure). Otherwise, we may attempt hemostasis by VATS; however, we constantly reconsider transitioning to thoracotomy if hemostasis is difficult to achieve. Such an algorithm should be refined according to each institution and surgical team because technical, instrumental, and human resources can differ, i.e., the presence of a cardiovascular team, availability of a blood-withdrawal system, or the amount of blood stocks.
Video cases: hemostasis by use of sealant
In our institute, we use TachoSil® Tissue Sealing sheet (CSL Behring, Tokyo, Japan) as a sealant, which is commonly used in Japan. TachoSil® is a sealant matrix containing human thrombin and fibrinogen. Upon contact with the bleeding site, the thrombin-fibrinogen reaction is activated, resulting in fibrin formation. TachoSil® and its predecessor product, TachoComb®, which contained bovine thrombin, have proven efficacy in hemostasis from various organs, including the pulmonary artery (9,10). We believe that achieving hemostasis using sealants should be attempted prior to conversion to thoracotomy, particularly when the bleeding occurs from areas where control is not always easier to achieve by thoracotomy. For example, bleeding from the intermediate pulmonary trunk may require isolation of the pulmonary artery at three sites: at the proximal site and at two distal sites, middle and lower pulmonary artery. Also, bleeding from vessels near the apex of the thoracic cavity is also difficult to visualize, clamp, and suture even after thoracotomy. In such cases, hemostasis by VATS using sealants may be quicker and easier.
Here we present two cases of VATS bleeding in which hemostasis was achieved using TachoSil®. One of the most common bleeding sites during VATS is the pulmonary artery, and we typically use TachoSil® for pulmonary artery bleeding (Figure 2, bleeding during right upper lobectomy) and occasionally for bleeding from systemic venous circulation (Figure 3, accessory hemiazygos vein). Upon bleeding, we first achieve temporary hemostasis by compression. We occasionally use adjacent organs for initial compression, such as an adjacent lung lobe during lung resection procedures or pericardial or mediastinal fat when the bleeding point is located near the anterior mediastinal area. During this initial compression period, we prepare for potential thoracotomy or transfusion and discuss methods of hemostasis within the team. If the location of the bleeding point is unclear, we refer to three-dimensional computed tomography images or immediately check the operation video. In our surgical team, we do not commonly select suturing for hemostasis during VATS because it can potentially aggravate the situation. Instead, we generally use sealants. After initial hemostasis by compression, we gently but quickly apply TachoSil® to the bleeding point. Although we cannot always stop bleeding with the sealant, we may still expect to reduce bleeding by compression with the sealant, as shown in Figure 3. Compression should not be too strong because it may enlarge the injury site, and it should be possible to feel the pulse of the pulmonary artery via the tip of the cotton stick. After several minutes of compression with TachoSil®, we slowly and gently remove the cotton stick with caution so as not to remove TachoSil® from the bleeding point. Application of lubricant jelly between the cotton stick and TachoSil® may prevent adhesion of the sealant to the cotton stick. We then apply additional TachoSil® to the bleeding site to achieve final hemostasis.


Conclusions
Bleeding during VATS is an important issue that ideally can be prevented. Although the incidence of bleeding during VATS may be low among skilled surgeons, it cannot be completely avoided, and we must be prepared for such situations. Infrastructure and surgical resources greatly differ among institutions and countries, but there are common issues in bleeding that can be shared, aiding in their prevention and in preparedness of thoracic surgeons. Here we reviewed our concept of VATS bleeding that we currently share within our surgical team. We believe that a key to safe surgery is to prepare for the worst and to strive for the best.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Visualized Surgery for the series “Emergency Response to Intraoperative Bleeding”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2018.11.07). The series “Emergency Response to Intraoperative Bleeding” was commissioned by the editorial office without any funding or sponsorship. SN and KS served as the unpaid Guest Editors of the series. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Masuda M, Kuwano H, Okumura M, et al. Thoracic and cardiovascular surgery in Japan during 2013: Annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2015;63:670-701. [Crossref] [PubMed]
- Shimizu K, Nakazawa S, Nagashima T, et al. 3D-CT anatomy for VATS segmentectomy. J Vis Surg 2017;3:88. [Crossref] [PubMed]
- Nakano T, Shimizu K, Nakano S, et al. Usefulness of three-dimensional computed tomographic angiography with bronchography for the planning of minimally invasive video-assisted thoracic surgery for intralobar pulmonary sequestration. Eur J Cardiothorac Surg 2013;43:199. [Crossref] [PubMed]
- Nagashima T, Shimizu K, Ohtaki Y, et al. An analysis of variations in the bronchovascular pattern of the right upper lobe using three-dimensional CT angiography and bronchography. Gen Thorac Cardiovasc Surg 2015;63:354-60. [Crossref] [PubMed]
- Nagashima T, Shimizu K, Ohtaki Y, et al. Analysis of variation in bronchovascular pattern of the right middle and lower lobes of the lung using three-dimensional CT angiography and bronchography. Gen Thorac Cardiovasc Surg 2017;65:343-9. [Crossref] [PubMed]
- Demmy TL, James TA, Swanson SJ, et al. Troubleshooting video-assisted thoracic surgery lobectomy. Ann Thorac Surg 2005;79:1744-52; discussion 1753.
- Gonzalez-Rivas D, Stupnik T, Fernandez R, et al. Intraoperative bleeding control by uniportal video-assisted thoracoscopic surgery. Eur J Cardiothorac Surg 2016;49:i17-24. [PubMed]
- Hsin MK, Yim AP. Management of complications of minimally invasive thoracic surgery. Respirology 2010;15:6-18. [Crossref] [PubMed]
- Ikeda T, Miyata Y, Tsutani Y, et al. Fibrinogen/thrombin-based collagen fleece (TachoComb(R)) promotes regeneration in pulmonary arterial injury. Eur J Cardiothorac Surg 2012;41:926-32. [Crossref] [PubMed]
- Agger P, Langhoff J, Smerup MH, et al. Comparison between TachoComb and TachoSil for surgical hemostasis in arterial bleeding: an animal experimental study. J Trauma 2010;68:838-42. [Crossref] [PubMed]
- Nakazawa S, Shimizu K, Kosaka T, et al. Hemostasis of bleeding from pulmonary artery. Asvide 2018;5:900. Available online: http://www.asvide.com/article/view/28730
- Nakazawa S, Shimizu K, Kosaka T, et al. Hemostasis of bleeding from accessory hemiazygos vein. Asvide 2018;5:901. Available online: http://www.asvide.com/article/view/28731
Cite this article as: Nakazawa S, Shimizu K, Kosaka T, Ohtaki Y, Mogi A, Shirabe K. Management of intraoperative bleeding during video-assisted thoracoscopic surgery. J Vis Surg 2018;4:245.


