Training program in robotic thoracic surgery
Introduction
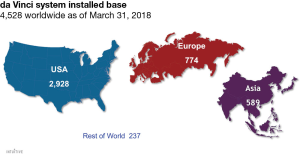
Since the introduction of current robotic-assisted surgery into surgical practice in 2000, the number of surgeries performed continues to increase. There are approximately 4,500 robotic platforms fully operational worldwide (Figure 1). Approximately half of these are located in the United States. The first reports describing the use of the robotic platform in thoracic surgery occurred in 2003 (1,2). In 2010, the Society of Thoracic Surgeons (STS) database reported that only a small number of lobectomies had been performed with robotic assisted thoracic surgery (RATS). By the end of 2013, 14% of all lobectomies were performed via robotic surgery (3). Oh et al. reported that the number of all lobectomies performed with RATS went from 7.6% in 2011 to 17.5% in 2015 (4). In that same year, over 6,000 robotic lobectomies were performed in the United States, and over 8,600 done worldwide (5). During the same period of time, the number of open lobectomies went from 54% to 43%, while the number of VATS lobectomies remained stable at 40%, suggesting an increased adoption of RATS by thoracic surgeons contributed to less open surgery (4). Here we present a pathway for proper adoption of robotic thoracic surgery.
RATS and VATS
Video assisted thoracic surgery (VATS) has been well accepted as a preferred method for surgical management of lung cancer. However, the adoption has been limited in part due to limited training of surgeons as well as technical demand of VATS in complex cases. The robotic platform offers a consistent and reliable high definition three-dimensional view, dashboard display of vital signs and instruments being used, a teaching console, wristed instruments with a greater degree of freedom enabling the surgeon to control the video-camera as well as multiple instruments simultaneously. Although the number of thoracic robotic surgery continues to increase (6-12), there exist a few limiting factors for safely adapting a RATS approach. One major barrier to adapting robotic surgery is the training of thoracic surgeons and his/her operating room (OR) team, availability of OR time distributed amongst other specialties, training residents, and fellows, as well as the difficulties encountered during the learning curve.
Training
A small number of training centers and proctors are available to teach RATS worldwide and it is encouraged to participate in a live case observation to understand the OR dynamic as well as the robotic techniques to determine if this approach is feasible for the aspiring surgeon. This often presents a challenge due to the costs and the required time away from the practice. Surgeons in training who decide to pursue robotic thoracic surgery could choose an academic center with an established robotic surgery training program and obtain certification offered by the manufacturer(s). Regardless of training/certification pathway chosen, it should include a few mandatory steps: (I) to obtain a firm knowledge of the robotic platform; (II) to develop the skills necessary to perform robotic surgery in the laboratory; (III) to be proctored by an surgeon with experience in RATS; (IV) to develop a surgical and anesthesia team whose familiarity with thoracic surgery is second to none; and (V) to attain the learning curve within a desirable/established period of time while monitoring patient outcomes
Knowledge of the robotic platform
The first phase of training involves learning the technical aspects of the robotic platform and the basic techniques of instrument control, instrument selection and camera management. This training is facilitated and is standardized by the manufacturer. During this initial step, the surgeon will be familiar with the equipment and the robotic system itself, he/she will know how to set up the operative field and dock the robot to the patient. At the end of the first step, the surgeon should have full knowledge of the robot’s operating system, the surgical cart, the surgeon’s console, and visual display. It is paramount to understand monopolar and bipolar energy in the robot since it requires foot and wrist action simultaneously.
Develop skills in the laboratory
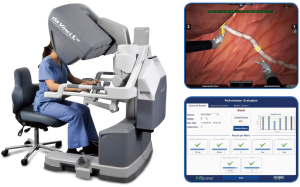
It involves robotic technical skills through laboratory simulation, didactic classes, and hands-on courses. The goal is to introduce the neophyte surgeon to a series of training modules and to proceed from a cautionary frame of mind to a faster and precise movement and hand-foot coordination utilizing robotic instruments. The training and simulation modules provide immediate feedback through a scoreboard. A score greater than 90% in each module indicates an adequate level of proficiency for each specific skill set (Figure 2). Simulation modules specific to thoracic surgery and pulmonary lobectomy are now available in some centers. Next, hands-on activities in the wet laboratory with either animal or cadaver models coupled with didactic courses will enable the surgeon to use different instruments, to dissect tissues applying monopolar or bipolar energy, and to handle tissue with the robotic instruments/arms as well as endo-suturing.
Although neither robotic nor video-assisted thoracic surgery provides tactile feedback, one would be required to develop visual cues to apply adequate force required to perform the operation without causing trauma to tissues. The application of bipolar energy for dissection requires early activation of the energy and pulling the tissue away from the dissection plane. Once these basic skills have been acquired, one should proceed to the third step (Figure 3).

Proctored by a surgeon with experience in RATS in the operative room. It is paramount that all staff members involved in the robotic team; surgeon, assistants, anesthesiologist, scrub technicians, and OR circulators should be well acquainted with the scheduled surgical procedure. At first, procedures performed with RATS would take longer than the average time for open or VATS procedures. As the learning curve is attained, communication amongst team members and surgeon’s versatility will improve, thus the time for each and every procedure will decrease. It is important to brief and debrief with the anesthesia and OR team to identify areas requiring improvement as well as to streamline the process and improve efficiency.
Develop a surgical and anesthesia team for RATS
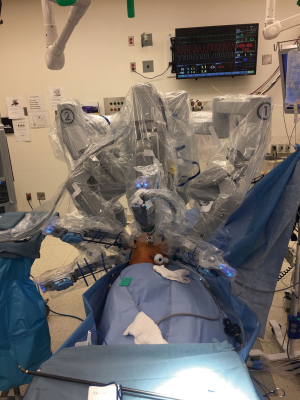
The team should be familiar with thoracic surgery. One must never compromise the patient’s safety for “efficiency” in the OR. At this stage, the surgeon should focus on the safety of the patient, the surgical technique itself, the positioning of the patient on the table in relation to the anesthesia cart, port placements, docking the robot, placing and exchanging instruments and the strategy for unexpected bleeding requiring conversion to open. The surgical team should incorporate all these during checklist and time-out. It is noteworthy once docking is completed, the anesthesiologist will have limited access to the patient’s double-lumen endotracheal tube. Correct placement of the ports and trocars should be done with the guidance of a 5 mm thoracoscope. Once desired trocars have been placed, docking is then carried out. When these two steps have been done correctly, there should be no collision of the robotic arms and no limitation of the movements with the instruments (Figure 4).
Attain the learning curve within a desirable/established period of time. Whenever unwanted bleeding is encountered, a planned and rehearsed strategy should be implemented without hesitation. Our approach is to have the assistant, at the bedside, apply gentle pressure over the bleeding area with sponged forceps as the surgeon moves swiftly towards the operative table while team members undock the robot and remove the instruments to convert to open thoracotomy. As the number of cases of anatomic pulmonary resections approaches 20, the efficiency of the operation starts to improve significantly (14-16). Each hospital credentialing process for robotic surgery is different, and some require different number of proctored cases from 2 to 5 followed by monitoring of the outcomes in the initial phase of the robotic program implementation. We suggest adopting a quality control and metrics spreadsheet for every RATS procedure from the start (Figure 5), and have scheduled, external audits of the program. We would encourage a more direct mentorship program with the proctoring surgeon to discuss troubleshooting, patient selection and technical pearls as the learning curve progresses.
Costs
There are many opportunities to control costs of surgical procedures overall. There are direct and indirect costs. Direct costs are charges related directly to patient care; chest CT scans, X-rays, medications, daily laboratory tests, dietary needs, the daily rate of hospital beds, OR equipment, disposable supplies, OR hourly rate, respiratory therapists, supplemental oxygen, chest tubes, benefits and salaried personnel who delivered care to the patients in and out of the OR and during hospitalization.
Indirect cost takes into account overhead costs, such as the price of the Robotic system, utilization, annual instruments maintenance contract, time away from clinical duties for personnel and team training, fitting or retrofitting of ORs, amortization of capital equipment and supplies, and the cost of utilities and administrative staff. Profit margin has been defined as the amount of reimbursed money by all payers subtracted by the total expenses of the patient encounter. The total expense is the sum of the direct and indirect costs over time. In 2014, Nasir et al. reported a median direct cost of $9,853, median indirect cost $5,587, and total expense per patient of $15,440, median Medicare reimbursement per patient of $18,937 with a median profit margin per patient of $3,497 (17). There are, however, local, regional and international variations, which should be taken into consideration when calculating expenses and profitability of RATS procedures. Most of the hospitals will distribute the indirect costs amongst different specialties. We should be mindful of the costs under our control, minimize unnecessary instrument exchanges, supplies and services (i.e., epidural catheters, central lines, etc.) supplies, prevent complications and facilitate appropriate hospital length of stay in order to ameliorate costs.
The introduction of a new modality for performing thoracic surgery will demand time and dedication. As one begins the perform RATS, it is tempting to fall back on the “old ways” whenever difficulties are encountered, may it be in the OR or outside. Another common misconceived idea is that of RATS is just VATS with a three-dimensional view and wristed instruments. In reality, it represents in some ways, a departure from video-assisted surgery into a different approach, which offers many technical improvements and state-of-art optics. It is expected that in the coming months, there are going to be many more improvements within the robotic platforms and compatibility with diagnostic procedures, catheter-based therapy, and radiology as well as distant learning.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Ricardo M. Terra and Paula A. Ugalde) for the series “Minimally Invasive Surgery - Robotics and VATS in Brazil” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2018.10.12). The series “Minimally Invasive Surgery - Robotics and VATS in Brazil” was commissioned by the editorial office without any funding or sponsorship. LH reports personal fees from INUITIVE SURGICAL, outside the submitted work, and INTUITIVE SURGICAL - HONORARIUM FOR PROCTORING AND SPEAKER BUREAU. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Morgan JA, Ginsburg ME, Sonett JR, et al. Advanced thoracoscopic procedures are facilitated by computer-aided robotic technology. Eur J Cardiothorac Surg 2003;23:883-7; discussion 887. [Crossref] [PubMed]
- Ashton RC Jr, Connery CP, Swistel DG, et al. Robot-assisted lobectomy. J Thorac Cardiovasc Surg 2003;126:292-3. [Crossref] [PubMed]
- Louie BE, Wilson JL, Kim S, et al. Comparison of Video-Assisted Thoracoscopic Surgery and Robotic Approaches for Clinical Stage I and Stage II Non-Small Cell Lung Cancer Using The Society of Thoracic Surgeons Database. Ann Thorac Surg 2016;102:917-24. [Crossref] [PubMed]
- Oh DS, Reddy RM, Gorrepati ML, et al. Robotic-Assisted, Video-Assisted Thoracoscopic and Open Lobectomy: Propensity-Matched Analysis of Recent Premier Data. Ann Thorac Surg 2017;104:1733-40. [Crossref] [PubMed]
- Linsky P, Wei B. Robotic lobectomy. J Vis Surg 2017;3:132. [Crossref] [PubMed]
- Seto Y, Mori K, Aikou S. Robotic surgery for esophageal cancer: Merits and demerits. Ann Gastroenterol Surg 2017;1:193-8. [Crossref] [PubMed]
- He H, Wu Q, Wang Z, et al. Short-term outcomes of robot-assisted minimally invasive esophagectomy for esophageal cancer: a propensity score matched analysis. J Cardiothorac Surg 2018;13:52. [Crossref] [PubMed]
- Cerfolio RJ, Ghanim AF, Dylewski M, Veronesi G, Spaggiari L, Park BJ. The long-term survival of robotic lobectomy for non-small cell lung cancer: A multi-institutional study. J Thorac Cardiovasc Surg 2018;155:778-86. [Crossref] [PubMed]
- Veronesi G, Park B, Cerfolio R, et al. Robotic resection of Stage III lung cancer: an international retrospective study. Eur J Cardiothorac Surg 2018;54:912-9. [Crossref] [PubMed]
- Wei S, Chen M, Chen N, Liu L. Feasibility and safety of robot-assisted thoracic surgery for lung lobectomy in patients with non-small cell lung cancer: a systematic review and meta-analysis. World J Surg Oncol 2017;15:98. [Crossref] [PubMed]
- Rückert JC, Swierzy M, Ismail M. Comparison of robotic and nonrobotic thoracoscopic thymectomy: a cohort study. J Thorac Cardiovasc Surg 2011;141:673-7. [Crossref] [PubMed]
- Keijzers M, de Baets M, Hochstenbag M, et al. Robotic thymectomy in patients with myasthenia gravis: neurological and surgical outcomes. Eur J Cardiothorac Surg 2015;48:40-5. [Crossref] [PubMed]
- Brito Filho F, Herrera L, DaSilva M. How to use robotic thoracic bipolar energy. Asvide 2018;5:839. Available online: http://www.asvide.com/article/view/28111
- Cheufou DH, Mardanzai K, Ploenes T, et al. Effectiveness of Robotic Lobectomy-Outcome and Learning Curve in a High Volume Center. Thorac Cardiovasc Surg. 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Özyurtkan MO, Kaba E, Toker A. What happens while learning robotic lobectomy for lung cancer? J Vis Surg 2017;10;3:27.
- Meyer M, Gharagozloo F, Tempesta B, et al. The learning curve of robotic lobectomy. Int J Med Robot 2012;8:448-52. [Crossref] [PubMed]
- Nasir BS, Bryant AS, Minnich DJ, et al. Performing robotic lobectomy and segmentectomy: cost, profitability, and outcomes. Ann Thorac Surg 2014;98:203-8; discussion 208-9. [Crossref] [PubMed]
Cite this article as: Brito Filho F, Herrera L, DaSilva M. Training program in robotic thoracic surgery. J Vis Surg 2018;4:229.