
A pragmatic view of the usefulness of video-mediastinoscopy in the modern era
“A method is described by which through a short incision in the suprasternal notch it is possible to dissect down along the whole length of the trachea and to palpate with a finger the immediately surrounding tissues. A specially designed instrument, resembling a children’s esophagoscope, is introduced into the passage thus dissected, and specimens from pathologic changes are excised or the tissues punctured with visual aid. The operation, which is performed under intubation anesthesia, has been used in over 100 cases without a complication.” —Carlens 1959
Introduction
It is well known that mediastinoscopy is losing attraction between thoracic surgeons, and surgeons are moving away from routine mediastinoscopy before lung cancer resection. The reason is the recent introduction of modern technologies such as endobronchial ultrasound (EBUS) guided transbronchial needle aspiration. Although reduced there are still indications to perform mediastinoscopy.
Video-mediastinoscopy (VM) allows a greater yield of lymph nodes with fewer complications and is therefore able to provide more accurate results and a negative predictive value superior to conventional cervical mediastinoscopy (CM) (1). CM is now rarely performed and we will refer to VM throughout the article.
The scope of this paper is two fold, we will describe in details our technique for VM, and clarify the current indications focusing on VM as a staging procedure.
Short history
The first report of exploration of the mediastinum through a suprasternal incision is attributed to Radner (2), who in 1955 removed lymph nodes from the mediastinum using his finger. CM was going to be described just few years later by Carlens (3), in 1959 in Sweden, who described the instrument and the procedure in more than 100 cases. Mediastinoscopy was further developed and popularized by Pearson, in the 1960s, in Canada (4,5). Mediastinoscopy gives access safely to most of the mediastinal lymph nodes bilaterally and represents an invaluable and irreplaceable tool in the staging of thoracic malignancy. Also, it has a value as a diagnostic operation, in case of mediastinal masses which can be biopsied, or even removed, from the superior and middle mediastinum during mediastinoscopy.
Ginsberg (6), probably inspired by a procedure described by Kirschner in 1971 (7), proposed, in 1987, the extended cervical mediastinoscopy that allows the biopsy of the lymph nodes in the aortopulmonary window and paraaortic region (stations 5 and 6) through the same classical cervical incision.
The introduction of the VM (8) by Coosemans et al. widened the relevance, the application and the safety of this procedure allowing more radical dissection of the mediastinum (9,10).
Indications
VM offers diagnosis for a range of benign and malignant conditions arising in the visceral mediastinum. Lymphomas, sarcoidosis and germ cell tumors are some examples. The volume of the specimens permits immunohistochemistry investigation. VM is largely offered prior to resection in the presence of proven or suspected thoracic malignancies and it directs the pathway of the patient affected by early or advanced stage lung cancer.
Staging mediastinoscopy
VM has a central role in the preoperative staging of patients affected by non-small cell lung cancer (NSCLC), as the management of the patients is driven by the mediastinal lymph nodes status. It is vital to rule out, with the highest confidence possible, the involvement of mediastinal nodes, as the patients with positive lymph nodes would be redirected to a multidisciplinary approach with eventual re-staging after upfront chemotherapy. On the other hand, the staging should involve less invasive procedures first, in order to minimize the morbidities and expedite the cancer pathway.
The revised guidelines for preoperative mediastinal lymph node staging for NSCLC from the European Society of Thoracic Surgeons (11) emphasized the use of VM. VM is supported by less invasive techniques: imaging, endoscopic and endobronchial ultrasound guided aspiration, but is not replaced entirely by them in daily practice. Similarly, the American College of Chest Physicians (ACCP) recommended EBUS-TBNA as the initial method to stage lung cancer (12).
Although VM has been considered risky in patients with superior vena cava syndrome and large goiter, some authors have proven its feasibility and safety (13-15).
VM vs. EBUS
Although there are few randomized controlled trials demonstrating the advantages of EBUS versus VM, it is evident that it is necessary to combine EBUS-EUS to obtain a sensibility and negative predicted value of 94% and 93% respectively. Navani et al. (16) in an open label, pragmatic, randomized controlled trial demonstrated that EBUS as a staging procedure increased survival compared with conventional diagnostic and staging tests (503 vs. 312 days). The tissue aspirated with a needle is considerably less than the entire lymph-nodes provided by VM, nevertheless, both EBUS-TBNA and esophageal ultrasound-guided fine needle aspiration techniques have been shown to provide sufficient material for molecular and DNA testing, extending their role beyond initial evaluation of the mediastinum. This helps to direct and personalize medical treatment and predict response to therapy. They are useful tools in order to confirm a radiological suspicion of N2, but they cannot be used to rule out lymph node involvement. In the future, assessing sonographic features of lymph nodes may become useful in predicting nodal metastasis, and the sensitivity of these techniques may improve.
VM in cN0 NSCLC
Many evidence confirm that the presence of enlarged lymph node on CT scan (1.0 cm in short axis and 1.5 cm in the long axis) is not accurately associated with neoplastic infiltration, and it leads to a diagnostic mislead in about 40% of cases. Furthermore, small lymph nodes can display metastatic tumour. De leyn et al. reported 20% positive nodes in a group of 235 patients with cN0. On the basis of their experience the authors suggest that all subjects should undergo mediastinoscopy (17).
Recently, is has been shown that prevalence of occult pN2–3 was only 8% when modern PET-CT pointed at clinical N0 NSCLC. None of the 5 verified definitions of centrality was predictive for occult pN2–3. However, each definition of centrality was related to any pN+ at a prevalence of 21%. The authors question if the indication for preoperative invasive mediastinal staging should be based on centrality alone (18).
VM in cN1 NSCLC
It is known that the likelihood of N2 disease increase at the presence of clinical N1 disease (18) and that the accuracy of PET-CT in revealing mediastinal nodes involvement is inconstant and decrease for primary tumors diameter larger than 3 cm (19,20). The above considerations prompt more invasive investigations in patient with a mass wider than 3 cm, or with clinical N1 disease or a nodule/mass not in the outer third of the lung. All these patients should undergo VM unless N2 disease has been proven already by EUS or EBUS. If EUS or EBUS are negative, the patient should then undergo mediastinoscopy to rule out a false negative.
VM in cN2 NSCLC
Modern guidelines published by societies such as European Society of Thoracic Surgeons (ESTS), National Institute for Health and Care Excellence (NICE), European Respiratory Society (ERS) recommend EBUS as the first staging method to biopsy N2 nodes in lung cancer patients to reduce unnecessary thoracotomies. This should provide a rate of unexpected N2 disease less than 10%. As thoracic surgeons we should prevent to bring in the operating room patients with unexpected N2 disease, especially in the presence of multi-station N2 involvement. Lee et al. demonstrated that the prevalence of N2 disease, in patients affected by early-stage NSCLC and negative mediastinum on PET and CT, was 2.9% if the primary tumor was in the outer third of the lung parenchyma, while this dramatically increased to 21.6% in central tumours (21). We also know that the likelihood of N2 disease increase with the presence of clinical N1 disease (19) and that the accuracy of PET-CT in revealing mediastinal nodes involvement is inconstant and decreases for primary tumor diameter larger than 3 cm (20,22,23). The above considerations mandate more invasive staging investigations in patients with a mass wider than 3 cm, or with clinical N1 disease or with a nodule/mass not in the outer third of the lung. All the above patients should undergo VM unless N2 disease has been proven already by EUS or EBUS. In case EUS or EBUS are negative, the patient should then undergo mediastinoscopy to rule out a false negative or differentiate multi station N2 from single station N2. The amount of tissue biopsied with a needle during ultrasound guided procedure is not comparable with the entire lymph nodes provided during mediastinoscopy. Therefore, the false negative rate is lower.
Mediastinoscopy and video-assisted thoracoscopic surgery (VATS)
Cervical mediastinoscopy presents important limitations since it is impossible to assess the aortopulmonary window and the para-aortic region. Also, the lymph nodes in the retro-subcarinal region are not biopsied. VATS assessment of the mediastinum alone or with cervical mediastinoscopy (13,24) gives a valid assistance in detecting mediastinal lymphadenopathies providing a complete view of the homolateral hemithorax, of the pleural cavity, and of the all others areas need to perform biopsies. Uniportal VATS (25,26), allows to assess azygos lymph nodes on the right hemithorax, while on left can assess the lymph nodes of the posterior mediastinum, along the distal segment of the left main bronchus, those lying in posterior subcarinal region in addition to hilar and paratracheal homolateral region, para-aortic lymph nodes and subaortic lymph nodes. Furthermore, thoracoscopy allows the exploration of the pulmonary ligament and para-oesophageal lymph nodes. VATS overcome the limitations of VM and classical Chamberlain operation at the cost of increased invasiveness.
Re-staging
Although supported by less evidence, VM or even re-mediastinoscopy (ReMs) can have a role in restaging of the mediastinum after induction therapy (chemotherapy or chemoradiotherapy). ReMs is feasible but the extent of fibrotic tissue reduces dramatically its accuracy when compared with primary staging VM. Also, the PET-CT, which established its superiority in the primary mediastinal staging over CT alone, has significantly decreased sensitivity after induction chemotherapy (27). De Leyn and colleagues (28), albeit on a small cohort of patients, demonstrated that PET-CT was superior in the restaging over ReMs. The results with restating VM and ReMs should be further evaluated (29-33).
Surgical technique
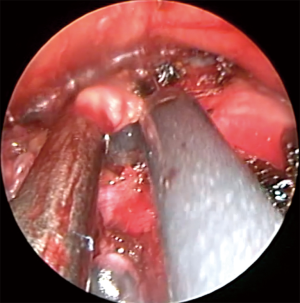
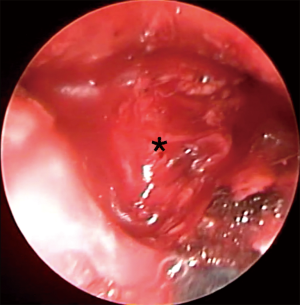
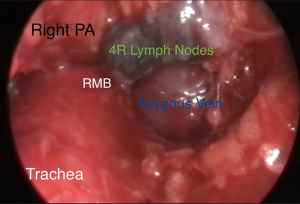
VM can be offered as a day surgery (34) or may require a single night of hospital stay. It is performed under general anesthesia maintained through a single lumen endotracheal tube. The patient is supine with the neck extended and a support under the shoulder. A 2.5-cm lower cervical incision is usually satisfactory to pass the mediastinoscope. The assistant or a small self-retainer retracts the wound in cephalic-caudal direction. The platysma is dissected and care should be paid to avoid the anterior jugular veins on each extremity of the incision. The strap muscles (sternohyoid first and then sternothyroid muscle) are visualized. The dissection now continues on a longitudinal fashion and through the connective tissue of the midline and avoiding the muscles. The edges of the muscles are retracted laterally to expose the deeper pre-tracheal fascia. The thyroid is sometime visualized and then needs retraction superiorly, which we perform with the index finger. The pre-tracheal fascia is retracted and opened with scissors, and then blunt finger dissection is performed over the trachea and into the mediastinum. It is important to be under the pretracheal fascia as this prevents vascular injuries. Solid mass or pathological lymph nodes may now be felt. The scope is passed and the endoscopic procedure starts (Figure 1). The operator moves to the head of the patient, the suction diathermy allows a precise mixture of sharp and blunt dissection and keeps the field clear, while the scope is used to offer counter traction. The trachea and the direction of the mediastinoscope are the landmarks to maintain the midline while penetrating deeper in the visceral mediastinum, and without diverting laterally in the paratracheal regions. The dissection continues until the right main and the left main bronchi are visualized and the subcarinal region is then exposed. A quite constant branch of the bronchial artery feeding the lymph node should be displayed over the carina and diathermy or clip should be applied to secure the haemostasis. This lymph node zone can now be exposed and biopsied. This anatomical region is defined, on the screen, by the pulmonary trunk superiorly, the medial aspects of the right and left main bronchi on each side. The assistant now holds the video-mediastinoscope and the surgeon can perform bimanual dissection to remove specimens (usually biopsy forceps and suction diathermy)(Figure 2). Attention is paid to avoid injury to the pulmonary artery and to the oesophagus. If the subcarinal region is emptied the oesophagus is clearly visible underneath (Figure 3). Retracting back the mediastinoscope, the lower and upper right paratracheal regions can be explored and biopsied (4R, 2R) as well as on the left side (4L, 2L). On the right side extreme attention should be paid to the azygos vein, which crosses over the right main bronchus to join the superior vena cava (Figure 4). Because it is not difficult to confuse the azygous vein for a 4R or 10 lymph nodes, it is sometime useful to test the lymph node with aspiration by a long needle. Injury to the azygous vein is the most common site of bleeding (36). The mediastinal pleura and the underlying right lung could be seen during the dissection, and care is needed to prevent a pneumothorax. On the left, the left pulmonary artery and the recurrent laryngeal nerve are the main structures to keep in mind during the dissection. The use of diathermy is minimized to prevent vocal cord dysfunction. The homeostasis is reviewed and the mediastinum can be packed for few minutes prior to closure. The strap muscles can be approximated with interrupted sutures.

Complications related to the use of mediastinoscopy
There is a paucity of evidence and lack of RCT to support VM’s superiority over CM (37). A review from Kirschner and colleagues in 1996 (38) shows that mediastinoscopy is an overall safe procedure with a reported mortality lower than 0.5% and a morbidity of 2.5%. The most common reported complications are transient recurrent nerve palsy, cervical incisional metastasis in 0.12% of cases, pneumothorax, esophageal perforation, tracheobronchial injury, gas embolisms, vascular mediastinal lesions. Another large experience with 2,137 mediastinoscopies (39) reported 12 complications (0.6%) and 1 deaths (0.05%). In the large series reported by Lemaire et al. (36), over nine years of consecutive experience with 2,145 procedures, the most common complication was vocal cord dysfunction (0.55%), followed by bleeding (0.33%) and pneumothorax in 0.09%. The bleeding from the pulmonary artery, after biopsy of a 4R lymph node, lead to death one patient (0.05% mortality). Protocols in place are required to manage bleeding during VM and senior expertise is essential (40).
Conclusions
VM remains a fundamental technique in thoracic surgery. It is a relatively quick procedure (30 to 60 minutes) which can be offered without hospitalization. It provides diagnosis for many thoracic conditions affecting the visceral mediastinum and it overcomes the weakness of the EBUS/EUS-FNBA for mediastinal staging of patients affected by NSCLC.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Tommaso Claudio Mineo) for the series “Mediastinal Surgery” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2018.06.08). The series “Mediastinal Surgery” was commissioned by the editorial office without any funding or sponsorship. MM serves as an unpaid editorial board member of Journal of Visualized Surgery from Dec 2016 to Nov 2018. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Leschber G, Sperling D, Klemm W, et al. Does video-mediastinoscopy improve the results of conventional mediastinoscopy? Eur J Cardiothorac Surg 2008;33:289-93. [Crossref] [PubMed]
- Radner S. Suprasternal node biopsy in lymphspreading intrathoracic disease. Acta Med Scand 1955;152:413-5. [Crossref] [PubMed]
- Carlens E. Mediastinoscopy: a method for inspection and tissue biopsy in the superior mediastinum. Dis Chest 1959;36:343-52. [Crossref] [PubMed]
- Pearson FG. Mediastinoscopy: a method of biopsy in the superior mediastinum. Canadian journal of surgery. Can J Surg 1963;6:423-9. [PubMed]
- Pearson FG. Lung cancer: the past twenty-five years. Chest 1986;89:200S-205S. [PubMed]
- Ginsberg RJ, Rice TW, Goldberg M, et al. Extended Cervical Mediastinoscopy. J Thorac Cardiovasc Surg 1987;94:673-8. [PubMed]
- Kirschner PA. Mediastinoscopy in superior vena cava obstruction. In: Jepssen O, Ruhbek-Soreneon H. editors. Mediastinoscopy: Proceedings of an International Symposium. Odense, Denmark: Odense University Press, 1971:40.
- Coosemans W, Lerut TE, Van Raemdonck DE. Thoracoscopic surgery: the Belgian experience. Ann Thorac Surg 1993;56:721-30. [Crossref] [PubMed]
- Martin-Ucar AE, Chetty GK, Vaughan R, et al. A prospective audit evaluating the role of video-assisted cervical mediastinoscopy (VAM) as a training tool. Eur J Cardiothorac Surg 2004;26:393-5. [Crossref] [PubMed]
- Hürtgen M, Friedel G, Toomes H, et al. Radical video-assisted mediastinoscopic lymphadenectomy (VAMLA)--technique and first results. Eur J Cardiothorac Surg 2002;21:348-51. [Crossref] [PubMed]
- De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:787-98. [Crossref] [PubMed]
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-e250S.
- Dosios T, Theakos N, Chatziantoniou C. Cervical mediastinoscopy and anterior mediastinotomy in superior vena cava obstruction. Chest 2005;128:1551-6. [Crossref] [PubMed]
- Mineo TC, Ambrogi V, Nofroni I, et al. Mediastinoscopy in superior vena cava obstruction: analysis of 80 consecutive patients. Ann Thorac Surg 1999;68:223-6. [Crossref] [PubMed]
- Migliore M, Costanzo M, Cannizzaro MA. Cervico-mediastinal goiter: is telescopic exploration of the mediastinum (video mediastinoscopy) useful? Interact Cardiovasc Thorac Surg 2010;10:439-40. [Crossref] [PubMed]
- Navani N, Nankivell M, Lawrence DR, et al. Lung cancer diagnosis and staging with endobronchial ultrasound-guided transbronchial needle aspiration compared with conventional approaches: an open-label, pragmatic, randomised controlled trial. Lancet Respir Med 2015;3:282-9. [Crossref] [PubMed]
- De Leyn P, Stroobants S, De Wever W, et al. Prospective comparative study of integrated positron emission tomography-computed tomography scan compared with remediastinoscopy in the assessment of residual mediastinal lymph node disease after induction chemotherapy for mediastinoscopy-proven stage IIIA-N2 non–small-cell lung cancer: a Leuven Lung Cancer Group Study. J Clin Oncol 2006;24:3333-9. [Crossref] [PubMed]
- Decaluwé H, Moons J, Fieuws S, et al. Is central lung tumour location really predictive for occult mediastinal nodal disease in (suspected) non-small-cell lung cancer staged cN0 on 18F-fluorodeoxyglucose positron emission tomography-computed tomography? Eur J Cardiothorac Surg 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Hishida T, Yoshida J, Nishimura M, et al. Problems in the current diagnostic standards of clinical N1 non-small cell lung cancer. Thorax 2008;63:526-31. [Crossref] [PubMed]
- Wang J, Welch K, Wang L, et al. Negative predictive value of positron emission tomography and computed tomography for stage T1-2N0 non-small-cell lung cancer: a meta-analysis. Clin Lung Cancer 2012;13:81-9. [Crossref] [PubMed]
- Lee PC, Port JL, Korst RJ, et al. Risk factors for occult mediastinal metastases in clinical stage I non-small cell lung cancer. Ann Thorac Surg 2007;84:177-81. [Crossref] [PubMed]
- Gómez-Caro A, Boada M, Cabañas M, et al. False-negative rate after positron emission tomography/computer tomography scan for mediastinal staging in cI stage non-small-cell lung cancer. Eur J Cardiothorac Surg 2012;42:93-100. [Crossref] [PubMed]
- Carnochan FM, Walker WS. Positron emission tomography may underestimate the extent of thoracic disease in lung cancer patients. Eur J Cardiothorac Surg 2009;35:781-4. [Crossref] [PubMed]
- Migliore M, Criscione A, Calvo D, et al. Minimal access anterior mediastinotomy. Updates Surg 2013;65:59-61. [Crossref] [PubMed]
- Migliore M. Efficacy and safety of single-trocar technique for minimally invasive surgery of the chest in the treatment of noncomplex pleural disease. J Thorac Cardiovasc Surg 2003;126:1618-23. [Crossref] [PubMed]
- Migliore M, Calvo D, Criscione A, et al. Uniportal video assisted thoracic surgery: summary of experience, mini-review and perspectives. J Thorac Dis 2015;7:E378-80. [PubMed]
- Hoekstra CJ, Stroobants SG, Smit EF, et al. Prognostic relevance of response evaluation using [18F]-2-fluoro-2-deoxy-D-glucose positron emission tomography in patients with locally advanced non-small-cell lung cancer. J Clin oncol 2005;23:8362-70. [Crossref] [PubMed]
- De Leyn P, Stroobants S, De Wever W, et al. Prospective comparative study of integrated positron emission tomography-computed tomography scan compared with remediastinoscopy in the assessment of residual mediastinal lymph node disease after induction chemotherapy for mediastinoscopy-proven stage IIIA-N2 non–small-cell lung cancer: a Leuven Lung Cancer Group Study. J Clin Oncol 2006;24:3333-9. [Crossref] [PubMed]
- Call S, Rami-Porta R, Obiols C, et al. Repeat mediastinoscopy in all its indications: experience with 96 patients and 101 procedures. Eur J Cardiothorac Surg 2011;39:1022-7. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Ojha B. Restaging patients with N2 (stage IIIa) non–small cell lung cancer after neoadjuvant chemoradiotherapy: A prospective study. J Thorac Cardiovasc Surg 2006;131:1229-35. [Crossref] [PubMed]
- Pauwels M, Van Schil P, De Backer W, et al. Repeat mediastinoscopy in the staging of lung cancer. Eur J Cardiothorac Surg 1998;14:271-3. [Crossref] [PubMed]
- Marra A, Hillejan L, Fechner S, et al. Remediastinoscopy in restaging of lung cancer after induction therapy. J Thorac Cardiovasc Surg 2008;135:843-9. [Crossref] [PubMed]
- Zieliński M, Hauer Ł, Hauer J, et al. Non-small-cell lung cancer restaging with transcervical extended mediastinal lymphadenectomy. Eur J Cardiothorac Surg 2010;37:776-80. [Crossref] [PubMed]
- Molins L, Fibla JJ, Pérez J, et al. Outpatient thoracic surgical programme in 300 patients: clinical results and economic impact. Eur J Cardiothorac Surg 2006;29:271-5. [Crossref] [PubMed]
- Migliore M, Nardini M, Rogers LJ, et al. Videomediastinoscopy. Asvide 2018;5:616. Available online: http://www.asvide.com/article/view/25840
- Lemaire A, Nikolic I, Petersen T, et al. Nine-year single center experience with cervical mediastinoscopy: complications and false negative rate. Ann Thorac Surg 2006;82:1185-9. [Crossref] [PubMed]
- Zakkar M, Tan C, Hunt I. Is video mediastinoscopy a safer and more effective procedure than conventional mediastinoscopy? Interact Cardiovasc Thorac Surg 2012;14:81-4. [Crossref] [PubMed]
- Kirschner PA. Cervical mediastinoscopy. Chest Surg Clin N Am 1996;6:1-20. [PubMed]
- Hammoud ZT, Anderson RC, Meyers BF, et al. The current role of mediastinoscopy in the evaluation of thoracic disease. J Thorac Cardiovasc Surg 1999;118:894-9. [Crossref] [PubMed]
- Park BJ, Flores R, Downey RJ, et al. Management of major hemorrhage during mediastinoscopy. J Thorac Cardiovasc Surg 2003;126:726-31. [Crossref] [PubMed]
Cite this article as: Migliore M, Nardini M, Rogers LJ, Vidanapathirana P, Dunning J. A pragmatic view of the usefulness of video-mediastinoscopy in the modern era. J Vis Surg 2018;4:145.


