Trileaflet aortic valve reconstruction using glutaraldehyde fixed autologous pericardium
Introduction
Aortic valve replacement (AVR) continues to be the gold standard for surgical treatment of calcific aortic stenosis. There are two basic artificial heart valves to choose from for replacement; however, both are not perfect. Bioprosthetic valves are made from biologic tissue mounted onto a frame. Generally, these types of valves offer the freedom from long-term anticoagulation but have poorer durability compared to mechanical valves. The problem with mechanical valves is the increased thrombogenicity, which results in patients requiring long-term anticoagulation (1).
Recently, there has been an influx of techniques to reconstruct the aortic valve with the hopes of improved durability and freedom from anticoagulation (2-6). Many of these techniques are not yet standardized and underutilized. One of the techniques that has shown promising long-term results is the trileaflet aortic valve reconstruction using glutaraldehyde fixed autologous pericardium initially championed by Shigeyuki Ozaki, MD (5).
This technique has begun to gain popularity in the United States. We have also employed this technique at our institution with promising results. In this report, we describe our approach for trileaflet aortic valve reconstruction using autologous pericardium.
Patient selection and workup
This technique is offered to patients undergoing AVR due to all aortic valve diseases including aortic stenosis and aortic insufficiency. Absolute exclusion criteria include lack of autologous pericardium, aortic root dilation and aortic sinus dilation. Patients are educated on this procedure, including the risks of the operation. The patients then sign an informed consent.
Pre-operative preparation
All patients undergo electrocardiogram, transthoracic echocardiogram, and cardiac catheterization. In addition to this, preferably, a CT of the chest without contrast is obtained to assess calcification of the aortic valve, though this is not a must.
Equipment preference
Standard equipment is used for this procedure. The use of the Ozaki VRec Sizer System is needed to create the neo-leaflets. This system comes with sizers and templates to sketch out the neo-leaflets. 0.6% glutaraldehyde is needed to fix the pericardium.
Procedure
The patient is anesthetized in the usual manner for cardiac surgery. The patient is then intubated with a single lumen endotracheal tube. Venous and arterial catheters are placed for hemodynamic monitoring. The transesophageal echocardiography probe is placed and a comprehensive exam is done. The patient is then prepped from the neck down to the knees. In concomitant coronary artery bypass, the patient is prepped down to the feet.
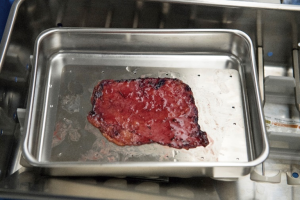
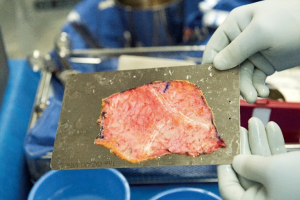
Median sternotomy is completed and the pericardium is exposed in the usual fashion. At this point, preparation of the autologous pericardium is then started by removing fat and redundant tissue on the outer surface of the pericardium (Figure 1). An approximate 8 cm × 8 cm piece of pericardium is removed and then promptly treated with 0.6% glutaraldehyde for 10 minutes. It is then washed with saline (Figure 2). Pericardial stay sutures are then placed to elevate the heart and to optimize exposure.
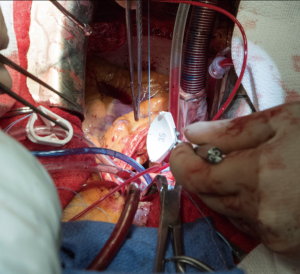
All the procedures were completed after cardioplegic arrest on cardiopulmonary bypass. After placing a double purse-string suture using 3-0 Ticron, an aortic cannula is placed, with sizing dependent on patient BSA. Venous cannulation is done using a dual-stage venous cannula through the right atrium through a single purse-string suture using 3-0 polypropylene. However, if a concomitant procedure is needed, then caval isolation is done and 2 stage venous cannulation used. A retrograde cannula is placed into the coronary sinus and once again secured using a purse-string suture with 3-0 polypropylene. Another 3-0 polypropylene purse-string is placed in the right superior pulmonary vein, use to secure a left ventricular vent catheter. An antegrade cardioplegia needle is inserted. The heart is arrested using retrograde and antegrade cardioplegia. The heart is maintained by retrograde cardioplegia every 15 minutes.
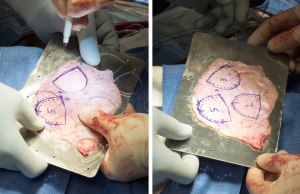
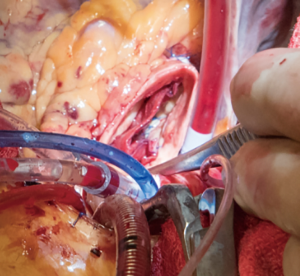
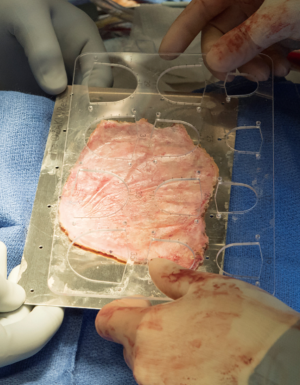
A transverse aortotomy is made approximately 1.5 cm from the right coronary ostium. After the aortotomy, the disease aortic leaflets are excised with Metzenbaum scissors and calcium removed with a ronger. The size of each commissure is then measured using sizers (Figure 3). These measurements will correlate to a template used to create the neo-leaflets from the pericardium (Figure 4). The correct leaflet size is sketched out onto the fixed glutaraldehyde-treated autologous pericardium and trimmed (Figure 5). The annular portion of the neo-leaflet is then sutured to the annulus using a 4-0 polypropylene suture in a running fashion (Figure 6). Commissures are then secured and coaptation is achieved using 4-0 polypropylene sutures as well (Figure 7). Once the valve reconstruction is completed, coaptation of the three cusps is checked by using saline and negative pressure on the left ventricular vent (Figure 8).
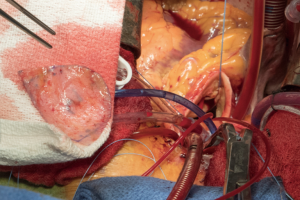
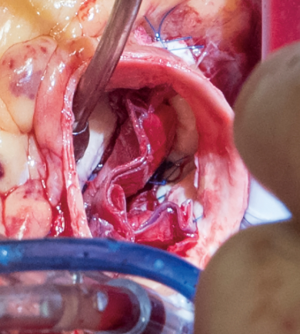
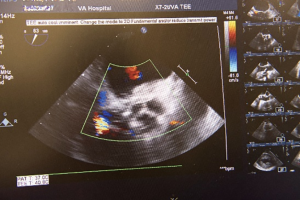
The aortotomy is closed using 4-0 polypropylene sutures. A Valsalva is done prior to tying the last running suture to de-air the ventricle and the aorta. At this point, the anesthesiologist uses the TEE to thoroughly evaluate the heart for air, ventricular function, and the function of the reconstructed valve (Figure 9). Additional de-airing maneuvers may be needed. Stenosis or regurgitation, perivalvular leak and coaptation of the leaflets are checked (Figure 10). Once the reconstructed valve looks satisfactory, cardiopulmonary bypass is discontinued. Protamine is given. The cardiopulmonary cannulas are removed once appropriate. Hemostasis is checked and two drains are placed, one in the anterior mediastinum and one behind the heart. The sternum is closed using steel wires and the wound is closed in layers.

Role of team members
Preoperative, intraoperative and post-operative care of the patient is overseen by the primary surgeon of the case. Surgical residents are generally present for these cases as well. The role of the surgical resident depends on the level of training. We have both an integrated 6-year cardiothoracic surgical residency program that includes residents coming directly out of medical school, and a traditional pathway in which the resident has completed general surgery residency. Generally, the resident will complete most of the initial stages of the case by doing the sternotomy and the dissection of the pericardium. The primary surgeon decides whether the pericardium is usable and the size needed. The primary surgeon will assist the trainee in the exposure and cannulation of the patient.
Cardiac anesthesiologist is used for all cases. The anesthesiologist and their resident will place arterial and venous cannulas for monitoring hemodynamics. They will also perform the intubation. Furthermore, it is important to have an anesthesiologist who can perform a complete TEE examination to assess the aortic valve. Not only will the dynamics of the aortic valve need to be communicated and discussed with the primary surgeon, but ventricular function must be assessed, along with other valvular dysfunction. The cardiac anesthesiologist will monitor the ventilation and pharmacologically modulate hemodynamics during the case.
Other members of the team are also integral. An experienced perfusion team is also needed to assist with cardiopulmonary bypass and maintain protection of the heart. The ICU team is the first line of defense for issues with the patient while the surgical team is in the operating room. They will also manage the hemodynamics, respiratory, pain and other issues of the patient as it happens while consulting the primary surgeon. The nursing staff are actively involved in all aspects of the patient’s care towards the goal of appropriate discharge. They are another line of defense if issues were to arise.
Post-operative management
The patient is transferred after the operation, intubated, to the cardiac intensive care unit (ICU). Though the patient can be extubated in the operating room, the patient is generally projected to be extubated within 4 hours of the operation in the ICU. During this time, pressor and inotropic support is weaned. Chest tube output is meticulously recorded to ensure hemostasis. Once extubated, PO intake starts with liquids only, and then a trial of regular food is started the next morning. The patient is transferred to cardiac step-down unit once hemodynamic stability, respiratory sufficiency and laboratory values have stabilized. Chest tubes are removed as early as possible once output is appropriate. Multimodal analgesic medications are given, which include patient controlled analgesia, narcotic medications and acetaminophen. The patient is encouraged to mobilize early as well with the assist of cardiopulmonary rehab specialists.
Since this procedure was developed in the last decade, follow-up is crucial. The patient should have a transthoracic ECHO done on the first follow-up visit and once again in one year to assess the mobilization of the leaflets.
Tips, tricks and pitfalls
Thorough pre-operative assessment of the patient is necessary. If there is suspected aortic root or sinus dilation, this operation should not be considered. Furthermore, autologous pericardium is used during this case. If there is any history of pericarditis or radiation to the chest that could compromise the pericardium, this operation should not be considered. Furthermore, this technique has been done for multiple pathologies related to the aortic valve. This technique has not only been done by our institution, but other institutions as well, for aortic stenosis, regurgitation and endocarditis. Sizing and commissural competence are both critical aspects of the procedure. Lastly, the resultant neo-leaflets have an increased coaptation zone as seen in video.
Conclusions
AVR continues to be the gold standard of treatment for aortic valve disease. However, due to the limitations of mechanical valve and bioprosthetic valve, there have been increasing number of techniques to reconstruct the aortic valve in hopes to address these limitations. Furthermore, there is currently no standardized reconstructive technique.
This technique has shown to have excellent durability and long-term hemodynamics. It is also a method in which reconstruction is standardized with the measurement of the commissures and the associated sized neo-leaflet construction. In the largest series that consisted of 416 consecutive patients, there were no conversions to prosthetic valve replacement. Peak pressure gradients one week and 5.5 years after surgery were excellent. The in-hospital mortality was 2%, with a freedom from reoperation of 96.7% with a 73-month follow-up. This study proves the safety and feasibility of the technique (5). However, this represents one institution and further studies of this kind need to reproduce this success before widespread adoption. Our experience and results using this technique show promise.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2018.06.09). DC reports personal fees from Japanese Organization for Medical Device Development, Inc., personal fees from Terumo Cardiovascular Systems Corporation, personal fees from The Osler Institute, personal fees from Wolters Kluwer Health, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol 2014;64:e1-76. [Crossref] [PubMed]
- Aicher D, Fries R, Rodionycheva S, et al. Aortic valve repair leads to a low incidence of valve-related complications. Eur J Cardiothorac Surg 2010;37:127-32. [Crossref] [PubMed]
- Gleason TG. Bicuspid aortic valve repair by complete conversion from "raphe'd" (type 1) to "symmetric" (type 0) morphology. J Thorac Cardiovasc Surg 2014;148:2862-8.e1-2.
- Lansac E, de Kerchove L. Aortic valve repair techniques: state of the art. Eur J Cardiothorac Surg 2018;53:1101-7. [Crossref] [PubMed]
- Ozaki S, Kawase I, Yamashita H, Uchida S, et al. Aortic valve reconstruction using autologous pericardium for aortic stenosis. Circ J 2015;79:1504-10. [Crossref] [PubMed]
- Schafers HJ, Bierbach B, Aicher D. A new approach to the assessment of aortic cusp geometry. J Thorac Cardiovasc Surg 2006;132:436-8. [Crossref] [PubMed]
- Chan PG, Seese L, Chan EG, et al. TEE showing competency of the reconstructed aortic valve. Asvide 2018;5:599. Available online: http://www.asvide.com/article/view/25673
Cite this article as: Chan PG, Seese L, Chan EG, Gleason TG, Chu D. Trileaflet aortic valve reconstruction using glutaraldehyde fixed autologous pericardium. J Vis Surg 2018;4:133.