Treatment of pulmonary nodule: from VATS to RATS
Introduction
The solitary pulmonary nodule (SPN) is defined as a single well-marginated spherical opacity with a diameter ≤3 cm, entirely surrounded by lung parenchyma and not associated with atelectasis, adenopathy or pleural effusion.
Throughout the last decade, the exponential utilization of computed tomography (CT) and the broad application of screening programs, have led to a noteworthy increase in the detection of SPN. Therefore, a great number of patients with indeterminate lung node will be found in the coming years. The probability of malignancy of a SPN depends on the imaging features of the lesion and on patient’s risk factors (1,2).
The distinction between benign and malignant lesions is mandatory given that, in case of lung cancer, the prognosis is associated with nodule size and the early detection is an important factor in decreasing mortality.
The nonsurgical diagnostic options in case of pulmonary lesions consist in transbronchial biopsy or CT-guided percutaneous needle aspiration. Unfortunately, in most cases, it is difficult to obtain a certain diagnosis with these techniques due to the location, small diameter or in the event of sub-solid nodule feature (3,4).
For the abovementioned reasons, the surgical resection of the node is often proposed both for definitive diagnosis and therapeutic procedure. The standard of surgical lung biopsy has recently moved from thoracotomy to minimally invasive approach and video-assisted thoracoscopic surgery (VATS) has become the favoured approach to resect SPN.
When compared with open approach VATS presents clear advantages, but the lack of tactile feedback and of bimanual palpation during thoracoscopic procedure make it difficult to localize non-peripheral, subsolid and small nodules.
In the last years, several techniques (preoperative and intraoperative) have been developed to overcome these limitations and to decrease the rate of conversion to open thoracotomy.
Preoperative techniques
Among the preoperative techniques, the most used are CT-guided hook wire insertion or the micro-coil, staining with methylene blue or radiotracer injection (99Tc-labeled human serum albumin micro/macrosphere solution). Both techniques have advantages and disadvantages. The most common complication encountered is the pneumothorax (PNX) its rate ranges from 18% to 35% after the hook wire insertion, from 25% to 33% after methylene blue staining and from 5% to 10% after radiotracer injection (5-10). A significant number of failure has been reported both for hook wire dislodgment (ranging from 2.9% to 47%) (7,10,11) and for diffusion of methylene blue on pleural surface (13%) (12). The hook wire dislodgment frequently occurs during the transport of the patient to operating theatre or during surgery, while inducing the PNX or during lung manipulation. The device displacement can result in an unsuccessful procedure and can lead to pulmonary hemorrhage (12–35%).
The diffusion of methylene blue on the visceral pleura surface, is frequently observed in smokers due to the anthracotic pigmentation of the lung, this causes an important number of localization failures (around 13%) and a high rate of conversion to thoracotomy. Furthermore, a delay between the dye injection and surgical procedure (recommended 3 h or less) significantly reduces the concentration of staining and then the visualization of marked area.
The radio-guided technique overcomes the limitations of the above-mentioned methods. The injection of 99Tc-labelled human serum albumin microsphere solution guarantees a good success rate with limited procedure-related complications (8,9,13). Moreover, this technique ensures adequate oncological margin thanks to the possibility, during the resection, to verify the absence of radioactivity underneath the stapler line. The most important limitation of this method is the relatively short half-life of the radionuclide (approximately 6 h), consequently the resection must be performed within few hours after its inoculation. To overcome this restriction, Grogan et al, instead of albumin microspheres, use 0.4 mL of macro-aggregated solution. This seems to ensure 18 hours of target zone radioactivity without increasing risks for the patient or health care professionals (14).
Intraoperative techniques
The principal intraoperative detection techniques are the digital palpation and the ultrasound-guided localization.
The finger localization of the lung nodules can be difficult depending on the type of parenchyma (e.g., emphysema) or due to the subsolid nature of the nodule.
Similarly, the most important limitation of the intraoperative ultrasound-guided technique is represented by the quality of lung parenchyma. In fact, given the absence of ultrasound propagation in presence of air, in case of incomplete lung collapse (unsuccessful selective intubation or emphysema) the successful rate of this technique decreases significantly in COPD patients or in small volume centers (15-18).
A newer technique to localize nodule is the electromagnetic navigational bronchoscopy (ENBL), in which a recent CT scan is employed planning a track to the lung surface and to the node for dye marker location. This method seems to have several benefits: it can be done directly in the operating theatre (avoiding the delay due to patient transportation and the requirement of interventional radiology staff with a consequently more flexible surgical scheduling), it allows the surgeon to perform a needle biopsy at the moment of localization, it has a lower rate of complications and lower radiation exposure compared to percutaneous identification techniques (19). On the opposite hand this method requires specialized equipment and does add extra-costs, therefore further prospective studies are needed to investigate the cost effectiveness of this technique (20).
Methods
We have retrospectively analysed data the of all patients with SPN who underwent lung resection with radio-guided technique between January 2010 and December 2015.
The nodules selected for radio-guided thoracoscopic surgery (RGTS) were those with a diameter <10 mm and/or a distance from the visceral pleura ≥10 mm (e.g., Figure 1). The lesions with a maximum diameter greater than 3 cm and a distance from the pleural surface greater than 4 cm were excluded.
Technique
We used a solution composed of 0.2 mL of 99Tc-labeled human serum albumin microspheres (5–10 MBq) and 0.1 mL of non-ionic contrast to mark the nodule by CT-scan. The choice of Technetium-99m is due to its relatively short half-life (about 6 hours) and consequently its low absorbed-radiation.
The solution was injected into the lung nodule using a 22-G needle under CT scan. Thanks to the presence of nonionic contrast in the solution, the effective node marking was confirmed by a control CT.
Afterward, the patient was transported into the operating theatre for a mini-invasive resection [VATS or robotic-assisted thoracoscopic surgery (RATS)] of the node. All the procedures were executed under general anesthesia with selective intubation through a double-lumen tube. The patient positioned in a lateral decubitus and a PNX induced. Then a 7- or 10-mm trocar was inserted into the 6th or 7th intercostal space along the mid-axillary line (camera port). In all patients, after an initial exploration of the chest cavity, a second incision (11–12 mm) was performed and a thoracoscopic hand-held 11-mm-diameter collimated gamma probe connected to a gamma ray detector unit (ScintiProbe MR 100, Pol.Hi.Tech., Carsoli, Italy) was introduced (Figures 2,3). The detector unit transformed gamma ray emissions into audio-visual signals, proportional to the perceived radioactivity.
The lung surface was scanned, starting far from the area of the lesion, to estimate the radioactive ground and to reset the system. Then the probe was moved close to the target area, looking for the greatest radioactivity value. Afterward, the endostapler was introduced and a wedge resection of the radioactive area was carried out. During resection, the gamma probe can be used to verify the absence of radioactivity underneath the stapler line. The specimen was then removed from the pleural cavity into an endoscopic bag and the presence of the nodule was verified.
In cases of suspicion of primary pulmonary cancer and if the patient had an adequate pulmonary reserve, an intraoperative frozen section examination was also performed.
Results
Out of a total of 772 patients with SPN, treated between 2010 and 2015 in our unit, we collected data of 175 patients who underwent RGTS (Table 1).
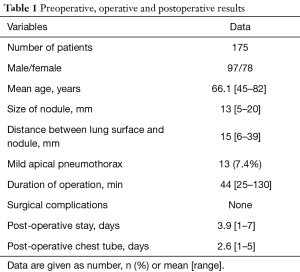
Full table
There were 97 men and 78 women, with a mean age of 66.1 years (range, 45–82 years). One hundred and twenty-one (69.1%) patients had had previous tumor. The mean diameter of nodes was 13 mm (range, 5–20 mm), and the mean distance from the visceral pleura was 15 mm (range 6–39 mm).
No significant procedure-related complications occurred, except for 13 patients (7.4%) who presented a PNX after the CT-guided injection. None of them required chest tube insertion. All patients underwent VATS resection within 3 h of the radiological procedure. The mean duration of surgical procedure was 44 min (range, 25–130 min).
The mean length of postoperative and pleural drainage stay were 3.9 days (range, 1–7 days) and 2.6 days (range, 1–5 days) respectively.
No postoperative mortality or perioperative complications occurred.
The successful rate of this procedure in localizing and resecting the SPN was around 100%.
Histopathological analysis showed 22 benign lesions (12.6%) and 153 malignant lesions (87.4%), which included 44 NSCLC (25.1%) and 109 metastases (62.3%) (Table 2). All patients affected by NSCLC and with an appropriate performance status, underwent a lobectomy or segmentectomy with systematic ilo-mediastinal lymphadenectomy. The surgical procedure was performed by open thoracotomy in 41 cases, by VATS in 3 cases, and using robotic surgery (da Vinci Surgical System, Intuitive Surgical, Inc., Sunnyvale, CA) in 11 cases.
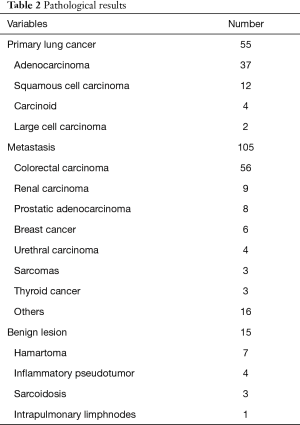
Full table
Discussion
A variety of localizing techniques are now available to detect small, subsolid or non-peripheral lung nodules that are difficult to pinpoint during minimally invasive procedures.
Several percutaneous and intraoperative techniques have been used to mark the lung nodes, those most applied are CT-guided staining with methylene blue, CT-guided hook-wire placement, CT-guided radiotracer injection and endothoracic ultrasonography. Recently the electromagnetic navigation bronchoscopy guided dye marking has also obtained increasing consents.
The percutaneous localising methods employ a CT scan executed before the surgery and have a communal and common complication, the pneumothorax.
The rate of PNX is 25–33% in patients who undergo methylene blue technique and 20–32% in those who undergo hook-wire placement method (5-7,9,10). Whereas, employing radiotracer injection, the PNX rate decreases significantly: in our experience it is around 7.5%, as well as date reported in literature (13,14).
Moreover, another aspect to take into consideration is the failure rate of each technique. Due to a diffusion of dye on the lung surface or an error in node localization, the failure rate of methylene blue method is nearly 13%, hence leading to a high rate of conversion to thoracotomy (5). The hook-wire placement is associated to a considerable incidence of dislodgement, ranging from 2.9% to 47% (7,10,11), that can also cause haemorrhage (12–35%) or pain (5–6%) (7,9).
The intraoperative ultrasound-guided localization technique does not require any preoperative procedures, but it is a decisively an operator-dependent method and it is difficult to perform in case of chronic obstructive pulmonary disease because it requires a totally deflated lung (15-18).
ENBL has also been exposed as a safe and effective technique to pinpoint small nodules (20-22). This method permits to surgical staff to perform the whole procedure in the operating theatre avoiding any delay caused by transport or the necessity of interventional radiology staff; it has a lower rate of complications and lower radiation exposure compared to percutaneous identification techniques and it also allows to perform a needle biopsy during localization (19,20,23). Nevertheless, ENBL is a newer technique and requires specialized paraphernalia and skilled staff (24).
Radio-guided technique has been shown to be an efficient and harmless technique in localizing deep and/or small nodules. This method has fewer complications and can upsurge the precision of the resection by verifying the absence of radioactivity underneath the stapler line (25).
Our experience has confirmed a high successful rate of RGTS (100%) with a lower incidence of complication (7.5%) compared to other technique.
During the latest phase of our experience, in case of suspected primitive lung cancer SPN, we have started to perform robotic resection of pulmonary nodes examined during the procedure. Then, in case of confirmation of malignant nature of the node, we perform robotic lobectomy/segmentectomy. In fact, we believe that robotic surgery for lung lobectomy offers several advantages both for the patient and for the surgeon. The advanced features of robotic platform and instruments offer to the surgeon a much-improved vision compared to VATS and open approach and allow the replication of the human wrist movements into the chest cavity, within minimally invasive intervention (26). Robotic lobectomy has shown a higher rate of lymph nodal upstaging, which is used as an indirect indicator of quality and radicality of surgical approach, compared to VATS and an equivalent rate compared with open surgery (27-32).
There are few studies on long-terms oncological outcomes of early stage patients treated with robotic approach, due to the relatively new development of this technique, still these studies are encouraging, showing an overall survival and disease-free survival comparable to open surgery (33,34).
Undoubtedly, in case of primitive lung cancer, the effective management and a quick diagnosis of SPN is the best chance for cure. Thanks to development of innovative techniques the diagnosis and treatment of pulmonary nodules that are generally considered difficult to localize can be obtain with minimally invasive approach, which guarantees both a good outcome and a better quality of life for the patient compared with open surgery.
This study shows that small and/or subsolid lung nodules can be safely marked using 99Tc-labelled human serum albumin microspheres and resected with minimally invasive surgery. This strategy, combined with robotic lobectomy and lymphadenectomy, can ensure a radical treatment in early stage lung cancer guarantying the best chance of cure and a good quality of life for the patient.
Acknowledgements
We thank Teresa Hung Key for linguistic accuracy checking.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This paper is a retrospective analysis of standard surgical procedures following the national ethical standards. The local R&D approved this report. There were no experimental therapied on the human subject, therefore, ethical committee evaluation and approval was not requested. All patients gave full consent regarding data collection and its use in the present study.
References
- Swensen SJ, Silverstein MD, Ilstrup DM, et al. The probability of malignancy in solitary pulmonary nodules. Application to small radiologically indeterminate nodules. Arch Intern Med 1997;157:849-55. [Crossref]
- Wahidi MM, Govert JA, Goudar RK, et al. Evidence for the treatment of patients with pulmonary nodules: when is it lung cancer?: ACCP evidence-based clinical practice guidelines (2nd edition). Chest 2007;132:94S-107S.
- Krochmal R, Arias S, Yarmus L, et al. Diagnosis and management of pulmonary nodules. Expert Rev Respir Med 2014;8:677-91. [Crossref]
- Raad RA, Suh J, Harari S, et al. Nodule characterization: subsolid nodules. Radiol Clin North Am 2014;52:47-67. [Crossref]
- Lenglinger FX, Schwarz CD, Artmann W. Localization of pulmonary nodules before thoracoscopic surgery: value of percutaneous staining with methylene blue. AJR Am J Roentgenol 1994;163:297-300. [Crossref]
- Eichfeld U, Dietrich A, Ott R, et al. Video-assisted thoracoscopic surgery for pulmonary nodules after computed tomography-guided marking with a spiral wire. Ann Thorac Surg 2005;79:313-6. [Crossref]
- Bernard A. Resection of pulmonary nodules using video-assisted thoracic surgery. The Thorax Group. Ann Thorac Surg 1996;61:202-4. [Crossref]
- Davini F, Gonfiotti A, Vaggelli L, et al. Thoracoscopic localization techniques for patients with solitary pulmonary nodule: radioguided surgery versus hookwire localization. J Cardiovasc Surg (Torino) 2006;47:355-9.
- Gonfiotti A, Davini F, Vaggelli L, et al. Thoracoscopic localization techniques for patients with solitary pulmonary nodule: hookwire versus radio-guided surgery. Eur J Cardiothorac Surg 2007;32:843-7. [Crossref]
- Chen S, Zhou J, Zhang J, et al. Video-assisted thoracoscopic solitary pulmonary nodule resection after CT-guided hookwire localization: 43 cases report and literature review. Surg Endosc 2011;25:1723-9. [Crossref]
- Ciriaco P, Negri G, Puglisi A, et al. Video-assisted thoracoscopic surgery for pulmonary nodules: rationale for preoperative computed tomography-guided hookwire localization. Eur J Cardiothorac Surg 2004;25:429-33. [Crossref]
- Kerrigan DC, Spence PA, Crittenden MD, et al. Methylene blue guidance for simplified resection of a lung lesion. Ann Thorac Surg 1992;53:163-4. [Crossref]
- Chella A, Lucchi M, Ambrogi MC, et al. A pilot study of the role of TC-99 radionuclide in localization of pulmonary nodular lesions for thoracoscopic resection. Eur J Cardiothorac Surg 2000;18:17-21. [Crossref]
- Grogan EL, Jones DR, Kozower BD, et al. Identification of small lung nodules: technique of radiotracer-guided thoracoscopic biopsy. Ann Thorac Surg 2008;85:S772-7. [Crossref]
- Kondo R, Yoshida K, Hamanaka K, et al. Intraoperative ultrasonographic localization of pulmonary ground-glass opacities. J Thorac Cardiovasc Surg 2009;138:837-42. [Crossref]
- Mattioli S, D'Ovidio F, Daddi N, et al. Transthoracic endosonography for the intraoperative localization of lung nodules. Ann Thorac Surg 2005;79:443-9. [Crossref]
- Ambrogi MC, Dini P, Boni G, et al. A strategy for thoracoscopic resection of small pulmonary nodules. Surg Endosc 2005;19:1644-7. [Crossref]
- Sortini D, Feo CV, Carcoforo P, et al. Thoracoscopic localization techniques for patients with solitary pulmonary nodule and history of malignancy. Ann Thorac Surg 2005;79:258-62. [Crossref]
- Bolton WD, Cochran T, Ben-Or S, et al. Electromagnetic Navigational Bronchoscopy Reduces the Time Required for Localization and Resection of Lung Nodules. Innovations (Phila) 2017;12:333-7. [Crossref]
- Abbas A, Kadakia S, Ambur V, et al. Intraoperative electromagnetic navigational bronchoscopic localization of small, deep, or subsolid pulmonary nodules. J Thorac Cardiovasc Surg 2017;153:1581-90. [Crossref]
- Makris D, Scherpereel A, Leroy S, et al. Electromagnetic navigation diagnostic bronchoscopy for small peripheral lung lesions. Eur Respir J 2007;29:1187-92. [Crossref]
- Leong S, Ju H, Marshall H, et al. Electromagnetic navigation bronchoscopy: A descriptive analysis. J Thorac Dis 2012;4:173-85.
- Bolton WD, Howe H 3rd, Stephenson JE. The utility of electromagnetic navigational bronchoscopy as a localization tool for robotic resection of small pulmonary nodules. Ann Thorac Surg 2014;98:471-5. [Crossref]
- Schwarz Y, Greif J, Becker HD, et al. Real-time electromagnetic navigation bronchoscopy to peripheral lung lesions using overlaid CT images: the first human study. Chest 2006;129:988-94. [Crossref]
- Ambrogi MC, Melfi F, Zirafa C, et al. Radio-guided thoracoscopic surgery (RGTS) of small pulmonary nodules. Surg Endosc 2012;26:914-9. [Crossref]
- Ricciardi S, Cardillo G, Zirafa CC, et al. Robotic lobectomies: when and why? J Vis Surg 2017;3:112. [Crossref]
- Veronesi G, Agoglia BG, Melfi F, et al. Experience with robotic lobectomy for lung cancer. Innovations (Phila) 2011;6:355-60. [Crossref]
- Merritt RE, Hoang CD, Shrager JB. Lymph node evaluation achieved by open lobectomy compared with thoracoscopic lobectomy for N0 lung cancer. Ann Thorac Surg 2013;96:1171-7. [Crossref]
- Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. [Crossref]
- Medbery RL, Gillespie TW, Liu Y, et al. Nodal Upstaging Is More Common with Thoracotomy than with VATS During Lobectomy for Early-Stage Lung Cancer: An Analysis from the National Cancer Data Base. J Thorac Oncol 2016;11:222-33. [Crossref]
- Martin JT, Durbin EB, Chen L, et al. Nodal Upstaging During Lung Cancer Resection Is Associated With Surgical Approach. Ann Thorac Surg 2016;101:238-44. [Crossref]
- Kent M, Wang T, Whyte R, et al. Open, video-assisted thoracic surgery, and robotic lobectomy: review of a national database. Ann Thorac Surg. 2014;97:236-42. [Crossref]
- Park BJ. Robotic lobectomy for non-small cell lung cancer (NSCLC): Multi-center registry study of long-term oncologic results. Ann Cardiothorac Surg 2012;1:24-6.
- Yang HX, Woo KM, Sima CS, et al. Long-term Survival Based on the Surgical Approach to Lobectomy For Clinical Stage I Nonsmall Cell Lung Cancer: Comparison of Robotic, Video-assisted Thoracic Surgery, and Thoracotomy Lobectomy. Ann Surg 2017;265:431-7. [Crossref]
Cite this article as: Davini F, Ricciardi S, Zirafa CC, Cavaliere I, Romano G, Melfi F. Treatment of pulmonary nodule: from VATS to RATS. J Vis Surg 2018;4:36.





