Uniportal video-assisted thoracic lung segmentectomy with near infrared/indocyanine green intersegmental plane identification
Introduction
The surgical approach to pulmonary lesions varies considerably on the basis of the underlying pathology and across various experiences.
The association of early detection of even smaller non-small cell lung cancer (NSCLC) (1) due to the diffusion of high-resolution computed tomography (HRCT), with the development of miniaturized advanced instrumentation for thoracic surgery, has led to a worldwide diffusion of minimally invasive thoracic surgery and to lung sparing procedures, not only in the field of benign lesions, but also in neoplastic pathologies.
Anatomical lung segmentectomies seem to represent the first-choice parenchyma-sparing procedure in the diagnosis of undetermined lung lesions and simultaneous effective treatment of selected patients affected by early stage NSCLC. Although more evidence is claimed definitively to establish the role of intentional segmentectomy for the surgical treatment of early NSCLC, a recent meta-analysis disclosed that segmentectomies produce similar survival compared to lobectomies for patients with stage I NSCLC (2).
Arguments in favour of lobectomy even for early stage NSCLC are based on the necessity of obtaining wide resection margins, in order to provide a lower recurrence rate with a longer disease free survival (DFS), especially in young patients. On the other hand, potential advantages of segmentectomy include preservation of lung function with lower morbidity and disability especially in elderly and low performance patients, and in case of synchronous or metachronous cancers.
A recently published paper (3) on patients undergoing lobectomy or segmentectomy showed that segmentectomy preserves whole lung function better than lobectomy, because it not only preserved the lobe but also increased the function of the ipsilateral non-operated lobe.
Despite these advantages, lung segmentectomies may represent a challenging procedure to be performed, especially by video-assisted thoracic surgery (VATS). They can be divided into two groups: typical segmentectomies, simple procedures such as right S1 and lingular segmentectomy (where parenchymal division involves 1 or 2 planes); and atypical ones, challenging procedures (such as S3 or S7, S8 segmentectomy) with the need of two or more intersegmental plane resections.
The identification of the intersegmental plain during segmentectomy is crucial to achieve the oncological proper excision with respect to adequate margins and the conservation of lung function. However, it still remains the most challenging step of the procedure, especially in chronic obstructive pulmonary disease (COPD) patients, where intersegmental plane identification and resection become more challenging due to the hyperinflated state of the parenchyma.
The historically most utilized technique in the identification of intersegmental plain during open procedures but also VATS is the creation of a demarcation border between target segment and residual parenchyma by reinflating the lung subsequently to the segmental bronchus closure (4).
The presence of collateral canals that permit retrograde inflation of the target segment, despite the closure of the segmental bronchus, may result in a difficult identification of the intersegmental line especially in COPD patients, where emphysematous lungs often get into an overinflated state. Several methods have been described in the effort to overcome these difficulties.
In 2007, Okada et al. proposed a bronchoscopic selective ventilation of the segmental bronchus (5), while Kamiyoshihara et al. (6) described the inflation of the involved segment only by instilling oxygen through a butterfly needle into the bronchus subtending the segment. In order to obtain the demarcation between inflated and deflated lung, but leaving inflated the target segment and not the residual lung, a slipknot bronchial ligation of the segmental bronchus during VATS segmentectomy was claimed by Oizumi et al. (7) in 2014. As alternative to lung inflating procedures, many authors evaluated the efficacy of dyes injection into the segmental pulmonary artery (8) or peripheral segmental bronchus following the ligation of the pulmonary vein (9,10). In 2009, an experimental study conducted on pigs (11) showed the feasibility of intersegmental plain identification under near infrared (NIR) imaging and indocyanine green (ICG) administration. ICG is an NIR fluorescent dye. The technique is based on the evaluation of blood supply to the lung by using the arterial segmentation instead of the traditional bronchial segmentation. ICG is administered intravenously (peripheral vein) after segmental artery ligation and segmental boundaries are evaluated under NIR thoracoscopy. The lung appears on the monitor as divided into two areas, blue and white, according to the blood flow (blue is the vascularized lung, white the devascularized). On the wave of this preliminary experience, Misaki and colleagues (12-14) reported their experience on patients underwent NIR thoracoscopy after intravenous ICG injection. This method, depending on blood flow and avoiding reinflating of the lung, resulted suitable not only for patients with normal parenchyma, but also and especially for emphysematous lungs.
Fluorescence imaging (FI) is commonly used in biomedical sciences (15) because it permits cell and tissue visualization both in vitro and in vivo. The principle of FI used in ICG angiography is simple: the tissue of interest is illuminated by a light at the excitation wavelength (about 750 to 800 nm) while it is observed at longer emission wavelengths (over 800 nm). ICG, commonly used in clinical practice for cardiac output determination, hepatic function and liver blood flow evaluation and ophthalmic angiography, has widely been adopted in surgery to help surgeons during, for example, hepatic, colonic and breast surgery. During lung segmentectomy, the NIR/ICG imaging system is of great utility in enabling surgeons to identify the intersegmental plain easily and without lung inflation. The usual dose for standard clinical use (0.1–0.5 mg/mL/kg) is very far from the toxicity level (>5 mg/kg of body weight). When administered intravenously, ICG is rapidly adsorbed by plasma proteins (lipoproteins) and just a minimal percentage reaches the interstitium. Half-life of ICG after intravenous injection is about 4 min, and it appears unconjugated in the bile in about 8–15 min after injection, without known metabolites (16,17). Contraindications to ICG injection are represented by history of allergy to iodides because of the risk of anaphylaxis. Adverse reactions as anaphylactic or urticarial reactions have been reported in patients with or without history of allergy to iodides.
On the basis of the encouraging results published on the use of NIR/ICG in intersegmental plain identification during thoracotomy (12), multiportal VATS (13,18) and robotic segmentectomy (19), we decided to apply this technique to uniportal VATS (U-VATS) segmentectomy.
Patient selection and workup
Patients affected by pulmonary nodule suspected to be an early stage NSCLC (stage I <2 cm), centrally located in a pulmonary segment with reasonably more than 2 cm of surgical margin are suitable for U-VATS lung segmentectomy as follow.
Preoperative work up consists of contrast enhanced HRCT scan, positron emission tomography and computed tomography (PET-CT) scan and lung function evaluation. Preoperative contrast enhanced HRCT scan is mandatory to evaluate carefully the vascular distribution to the target segment.
A careful history of the patient is got, with special attention to exclude contraindications to ICG injection as allergic diathesis to iodides, thyroid gland diseases, renal or liver failure, pregnancy or breastfeeding.
Procedure
U-VATS segmentectomy is performed under general anaesthesia with selective lung ventilation using a double-lumen endotracheal tube.
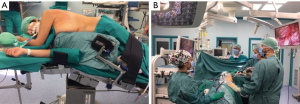
Patient’s position is a lateral, slightly wedge-shaped decubitus (Figure 1A). During operation, surgeon and assistant stand at the abdominal side of the patient, looking at the same monitor while the scrub nurse stands on the other side opposite to the operating surgeon, looking at a second monitor (Figure 1B).
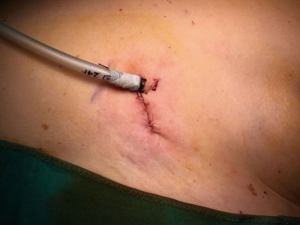
The surgical incision (3−4 cm in length) is created in the fourth or fifth intercostal space at the anterior axillary line. A wound protector is routinely used, and no distraction devices are employed to separate the ribs. A 30° 10-mm video thoracoscope and high-definition imaging system (Modular IMAGE1 S™ Camera Platform from KARL STORZ, Tuttlingen, Germany) are used together with dedicated thoracoscopic instrumentation. Major vessels are resected by endostaplers while small caliber vessels are usually sutured and divided using sealing devices and/or clips.
Segmental bronchial resection and closure can be achieved by endostapler or manual running suture.
Intersegmental plane identification by ICG
After ligation and transection of segmental vessels and bronchus, the camera is switched from standard white light to NIR light mode via a footswitch.
Five to 7-mL bolus of ICG (2.5 mg/10 mL) is intravenously (peripheral vein) administered depending on the body weight of the patient and parenchyma status. More ICG is needed in patients affected by emphysema to clearly visualize the intersegmental plain. NIR imaging is started immediately before the application of the contrast agent to gain a perfect dynamic and well-contrasted view of the boundaries between vascularized and non-vascularized lung segments. Immediately after ICG injection, we administer a 10-mL bolus of saline solution as suggested by Pardolesi and colleagues (19).
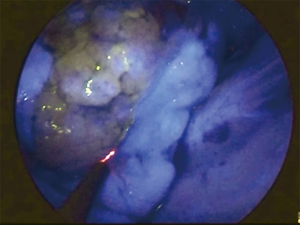
In about 30–40 seconds after injection and during NIR visualization, the segment to be resected appears “light grey” while the rest of the lung “switch on” in blue/green (Figure 2). Maximum green intensity is gained in about 1 min, then decreases. The limits of the segment to be resected are marked by electrocautery during NIR visualization (Figure 3).
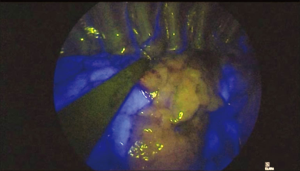
When necessary, ICG can be administered a second time up to a maximum dose of 5 mg/kg that is considered the maximum dosage before toxicity.
The marked intersegmental plane is resected by stapling.
The specimen is retrieved by a plastic bag through the incision wound.
Conventionally, systematic mediastinal lymph node dissection is performed after segmentectomy in case of cancer.
All patients undergoing U-VATS procedures receive intercostal nerve blockade with 3 mL of ropivacaine (4.75 mg/mL) per each intercostal space and a 24/28 Fr chest tube is inserted at the end of the procedure, through the same incision, in the posterior side (Figure 4).
Patients are usually extubated immediately after the operation in the operating room.
The chest drain left in place during U-VATS segmentectomies is usually removed on postoperative (PO) day 3 and the patient discharged immediately after.
Patients’ description
We herein describe the cases of 2 patients affected by PET-positive lung nodules with maximum diameter less than 2 cm (1 with lung metastasis, 1 with suspected primary lung cancer). Both patients were scheduled to undergo segmentectomy by U-VATS.
Patient 1 underwent right upper segmentectomy (Figure 5) and patient 2 underwent upper segmentectomy of the left lower lobe (Figure 6). ICG was injected into a peripheral vein after hilar transection of the target segment. Under NIR excitation the lung appears on the monitor as clearly separated into two areas, “light grey” (target segment-devascularized) and blue/green (rest of the lung), according to the blood supply.


Conclusions
The NIR/ICG visualization during U-VATS anatomical segmentectomy, avoiding lung reinflation and providing a rapid very clear demarcation of the intersegmental plain, has shown to be safe and very helpful in making the procedure easier and faster.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patients for publication of this manuscript and any accompanying images.
References
- National Lung Screening Trial Research Team, Church TR, Black WC, et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med 2013;368:1980-91. [Crossref] [PubMed]
- Bedetti B, Bertolaccini L, Rocco R, et al. Segmentectomy versus lobectomy for stage I non-small cell lung cancer: a systematic review and meta-analysis. J Thorac Dis 2017;9:1615-23. [Crossref] [PubMed]
- Nomori H, Shiraishi A. Cong Y, and al. Differences in postoperative changes in pulmonary functions following segmentectomy compared with lobectomy. Eur J Cardiothorac Surg 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Watanabe A, Ohori S, Nakashima S, et al. Feasibility of video-assisted thoracoscopic surgery segmentectomy for selected peripheral lung carcinomas. Eur J Cardiothorac Surg 2009;35:775-80. [Crossref] [PubMed]
- Okada M, Mimura T, Ikegaki J, et al. A novel video-assisted anatomic segmentectomy technique: selective segmental inflation via bronchofiberoptic jet followed by cautery cutting. J Thorac Cardiovasc Surg 2007;133:753-8. [Crossref] [PubMed]
- Kamiyoshihara M, Kakegawa S, Morishita Y. Convenient and improved method to distinguish the intersegmental plane in pulmonary segmentectomy using a butterfly needle. Ann Thorac Surg 2007;83:1913-4. [Crossref] [PubMed]
- Oizumi H, Kato H, Endoh M, et al. Slip knot bronchial ligation method for thoracoscopic lung segmentectomy. Ann Thorac Surg 2014;97:1456-8. [Crossref] [PubMed]
- Sugimoto S, Oto T, Miyoshi K, et al. A novel technique for identification of the lung intersegmental plane using dye injection into the segmental pulmonary artery. J Thorac Cardiovasc Surg 2011;141:1325-7. [Crossref] [PubMed]
- Oh S, Suzuki K, Miyasaka Y, et al. New Technique for Lung Segmentectomy Using Indocyanine Green Injection. Ann Thorac Surg 2013;95:2188-90. [Crossref] [PubMed]
- Sekine Y, Ko E, Oishi H, et al. A simple and effective technique for identification of intersegmental planes by infrared thoracoscopy after transbronchial injection of indocyanine green. J Thorac Cardiovasc Surg 2012;143:1330-5. [Crossref] [PubMed]
- Misaki N, Chang SS, Gotoh M, et al. A novel method for determining adjacent lung segments with infrared thoracoscopy. J Thorac Cardiovasc Surg 2009;138:613-8. [Crossref] [PubMed]
- Misaki N, Chang SS, Igai H, et al. New clinically applicable method for visualizing adjacent lung segments using an infrared thoracoscopy system. J Thorac Cardiovasc Surg 2010;140:752-6. [Crossref] [PubMed]
- Tarumi S, Misaki N, Kasai Y, et al. Clinical trial of video-assisted thoracoscopic segmentectomy using infrared thoracoscopy with indocyanine green. Eur J Cardiothorac Surg 2014;46:112-5. [Crossref] [PubMed]
- Kasai Y, Tarumi S, Chang SS, et al. Clinical trial of new methods for identifying lung intersegmental borders using infrared thoracoscopy with indocyanine green: comparative analysis of 2- and 1-wavelength methods. Eur J Cardiothorac Surg 2013;44:1103-7. [Crossref] [PubMed]
- Zhou Y, Kim YS, Milenic DE, et al. In vitro and in vivo analysis of indocyanine green-labeled panitumumab for optical imaging-a cautionary tale. Bioconjug Chem 2014;25:1801-10. [Crossref] [PubMed]
- Alander JT, Kaartinen I, Laakso A, et al. A review of indocyanine green fluorescent imaging in surgery. Int J Biomed Imaging 2012;2012:940585. [Crossref] [PubMed]
- Ishizawa T, Fukushima N, Shibahara J, et al. Real-time identification of liver cancers by using indocyanine green fluorescent imaging. Cancer 2009;115:2491-504. [Crossref] [PubMed]
- Tarumi S, Yokomise H. Video-assisted Thoracoscopic Segmentectomy Using Infrared Thoracoscopy with Indocyanine Green. Kyobu Geka 2016;69:671-5. [PubMed]
- Pardolesi A, Veronesi G, Solli P, et al. Use of indocyanine green to facilitate intersegmental plane identification during robotic anatomic segmentectomy. J Thorac Cardiovasc Surg 2014;148:737-8. [Crossref] [PubMed]
- Meacci E, Nachira D, Congedo MT, et al. Patient 1 underwent right upper segmentectomy. Asvide 2018;5:033. Available online: http://asvidett.amegroups.com/article/view/22423
- Meacci E, Nachira D, Congedo MT, et al. Patient 2 underwent upper segmentectomy of the left lower lobe. Asvide 2018;5:034. Available online: http://asvidett.amegroups.com/article/view/22425
Cite this article as: Meacci E, Nachira D, Congedo MT, Chiappetta M, Petracca Ciavarella L, Margaritora S. Uniportal video-assisted thoracic lung segmentectomy with near infrared/indocyanine green intersegmental plane identification. J Vis Surg 2018;4:17.