Uniportal video-assisted thoracoscopic surgery (VATS) sleeve resections for non-small cell lung cancer patients: an observational prospective study and technique analysis
Background
Bronchus sleeve resection for operative treatment of non-small cell lung cancer (NSCLC) is a gold standard in modern thoracic surgery in cases of centrally located tumors or hilar lymph node metastases. The lung sparing technique minimizes perioperative morbidity and mortality in comparison to pneumonectomy with equal oncological results and superior long-term life quality reports (1-3). At the early onset of bronchus sleeve resections, the vast majority of thoracic surgeons favored a posterolateral access for bronchial reconstructions due to the excellent exposition of the carinal area and to adequate space for surgical maneuvers (4). Because of the severe soft tissue trauma and respectable rib spreading required for this access and by the process of gaining experience with bronchial reconstruction, the anterolateral thoracotomy and the anterior hilum preparation became also commonly favorable for most surgeons for sleeve procedures (5,6). Parallel to the above surgical evolution, techniques of video assisted thoracoscopic surgery were widely adopted and anatomical resections for small, peripherally located tumors became also standard in high volume thoracic centers (7-10). Thoracoscopic techniques evolved rapidly due to promising clinical results concerning postoperative pain and perioperative morbidity (11). Advanced instruments and growing surgical experience allowed surgeons to reduce the required incisions (from 3-Port to uniportal) and to resect larger and more centrally located malignancies minimal invasively (12,13). It is a logical and expected advance in thoracic surgery that video-assisted thoracoscopic surgery (VATS) would be ultimately used also for complex bronchial resections. We therefore present in this study our early clinical results and technique of uniportal sleeve resections for patients with centrally located NSCLC or carcinoids.
Methods
Inclusion and exclusion criteria for uniportal VATS sleeve procedures were as following:
- Histologically proven NSCLC (including carcinoid tumours);
- Tumor size no larger than 5–6 cm;
- Tumor and nodal staging preoperatively by means of: CT-thorax, PET-CT or whole-body magnetic resonance imaging (MRI), rigid bronchoscopy with endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) or mediastinoscopy;
- Cases of N2 disease were excluded;
- No prior anatomical resection with hilum preparation on the side to operate on;
- No obesity with BMI >28 kg/m2;
- No prior radiation (neoadjuvant chemotherapy was not an exclusion criteria).
In terms of operative technique, a 4-cm uniportal incision was made in the 4th intercostal space anteriorly. No rib spreading was used and a soft tissue retractor was applied. Dissection was conducted in a usual manor using cautery or high energy devices. Staplers or sutures were used for dissection of the vascular structures and parenchyma. After extraction of the specimen radical lymphadenectomy in regions 2, 4, 7, 8 and 9 on the right side and 4, 5, 6, 7, 8 and 9 on the left side found place.
We adopted the same operative technique for uniportal VATS bronchial surgery as in open surgery in our institution. The bronchial tree was sharply dissected in the level of dissection taking care not to use cautery or high energy devices near the bronchial stumps so that areas of necrosis near the anastomosis could be avoided. The distal bronchial stump was left short near to the level of the parenchyma. Anastomosis was sutured in the same technique as in open surgery in our institution using a continuous, circular, monofilament absorbable or non-absorbable suture armed with two needles (poly-p-dioxanone or polypropylene, 90 cm, 4-0 or 0, 15-0, 2 mm). After completion no intercostal suture was used and adaptive sutures of the serratus muscle, the subcutis and of the skin found place. Drainage was inserted at the anterior border of the uniportal incision. All patients received inhalative antibiotic treatment (2×80 mg gentamycin) for 7 days according to our intern algorithm for sleeve resections and postoperative management (14) (Table 1). On the 7th postoperative day a flexible bronchoscopy was used to document the healing quality of the bronchial anastomosis according to our bronchus healing scale (15). In case of grade 1 or 2 anastomosis, the patient was discharged. Postoperative pain was documented twice a day until day of discharge using the numeric rating scale (NRS).
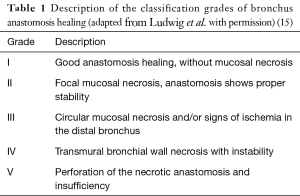
Full table
A video with the most important parts of a uniportal VATS left upper lobe intrapxericardial sleeve resection depicts the above procedure (Figure 1).

Results
In the period 2015–2017, n:40 patients with NSCLC were found eligible for uniportal VATS sleeve resection in our institution. In two cases a thoracotomy conversion because of severe hilar scar tissue was necessary. In 38 cases, a uniportal VATS sleeve resection could be completed. The perioperative data of the above collective are depicted in Table 2. No anastomotic insufficiencies could be documented. Complete R0-resection was achieved in 37 patients. One patient with preoperatively non-diagnosed N2-disease had a perineural invasion and a R1-status. In two patients, severe perioperative complications (one case of pulmonary embolism and one case of contralateral pneumonia) could be documented.
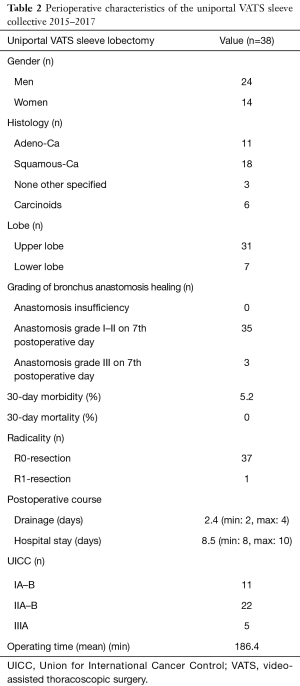
Full table
Conclusions
First reports of thoracoscopic sleeve lobectomies appeared in 2002 and 2007 (17,18). McKenna et al. presented in 2008 the first small series of thoracoscopic sleeve resections (19), other series has been published until today (20,21). In this series of patients, the procedure was limited to carcinoids or small centrally located NSCLC. In most cases the authors emphasized that VATS bronchoplastic procedures are feasible but highly advanced operative techniques are needed and a substantial experience in VATS anatomic resections is obligatory for safe results. With the introduction of uniportal VATS, the reports of thoracoscopic broncho- and angioplastic procedures increased after 2012 significantly, depicting the advantage of anterior visualization and dissection of the hilum (22-27). The techniques used for suturing the anastomosis varied according to operating institution. Meanwhile many case series of even more complex thoracoscopic resections (i.e., double sleeve) are being continuously reported (28).
Standard open bronchus sleeve lobectomy was conducted in our institution via anterolateral thoracotomy. Bronchial anastomosis was sutured using a circular, continuous resorbable 4-0 or 3-0 suture. All patients receive a 7-day 2×80 mg gentamycin prophylactic inhalation after bronchial sleeve resection and flexible bronchoscopy for grading of the local anastomosis healing was conducted after the 7-day inhalation (14,15,29). After introduction of VATS lobectomy in our institution and acquisition of a significant amount of operative experience in thoracoscopic techniques and instrumentation in advanced cases we began to indicate thoracoscopic sleeve resections for selected patients (mostly carcinoids). Initially, a 3-port technique was our standard VATS access. For the bronchus anastomosis, the same suture and technique was used like in our open surgery technique. In 2013, we adopted the uniportal access for VATS anatomical resections acknowledging the anterior camera view for hilar dissection. The learning curve for uniportal VATS was relatively short because of the former VATS lobectomy experience and of our standard anterolateral access for open surgery, which correlates perfectly with the uniportal technique.
After the initial uniportal VATS sleeve lobectomies experiences, we realized that a high number of factors could positively affect operating time and operative results. First is preoperative selection of the patients with our experience in open surgery analyzing the enhanced CT scans and preoperative bronchoscopy by the thoracic surgeon. This experience resulted in a small number of conversions. Although the exact location of the 4-cm skin incision was in our opinion not of significant importance for standard thoracoscopic lobectomies-segmentectomies and lymph node dissection (4th or 5th or even 6th intercostal space), this was crucial for uniportal sleeve resections. The 4th intercostal space (anteriorly) allows easier angle for anastomosis suture and reduces “overcrowding” or “clashing” of instruments. Primarily we adopted our open surgery technique using a poly-p-dioxanone suture for anastomosis. Although our results with poly-p-dioxanone showed excellent healing results on the 7th postoperative day, we intraoperatively realized that polypropylene sutures in contrast to poly-p-dioxanone runs smoother through the bronchial tissue, e.g., cartilage. Therefore, the use of polypropylene allows the adaptation of the bronchial edges only at the end of the anastomosis-suturing procedure. This minimized significantly our operating time without affecting negatively our healing results. The use of thoracoscopic instruments with shaft no longer than 20 cm also enhanced our ability to operate minimal invasively via the access incision. A curved tip needle holder proved to be extremely helpful, especially for the difficult posterior stiches through the anterior access. The use of various high energy devices for lymph node dissection and hilar exposition also affected our operating times positively. Gaining more experience with the procedure, we could reduce the required instruments to an absolute minimum (Figure 2). Initial operating times of >200 minutes could be easily reduced to <160 minutes.
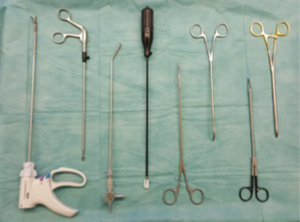
The main compromising factor for uniportal VATS sleeve resections proved to be tumor size. Malignancies with a diameter of >6 cm turned out to be initially extremely challenging and needing a larger incision than 4 cm for extraction. Infiltration of the pulmonary artery and angioplastic maneuvers though challenging did not pose an obligatory indication for conversion. The pulmonary artery could be easily dissected centrally via the anterolateral access and a tourniquet allowed the operating surgeon an equal exposure and operating safety as in open surgery.
Evaluating all available published data for thoracoscopic sleeve procedures via uniportal access or multiportal techniques we realize that a new era in minimal invasive thoracic surgery is arising though controlled randomized data are scarce. High volume thoracic centers report small series of even more advanced techniques for major bronchial resection (i.e., non-intubated sleeve lobectomies or carinal resections) (30). Robotic assisted thoracoscopic sleeve surgery could also play a significant role in advanced operating techniques making use the multi-angle operating skills of the robotic arms (31). We believe that uniportal sleeve resections are the logical evolution of VATS allowing patients with locally advanced malignancies to have quicker recovery and reduced perioperative pain.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from the patients for publication of this manuscript and any accompanying images.
References
- Massard G, Kessler R, Gasser B, et al. Local control of disease and survival following bronchoplastic lobectomy for non-small cell lung cancer. Eur J Cardiothorac Surg 1999;16:276-82. [Crossref] [PubMed]
- Ludwig C, Stoelben E, Olschewski M, et al. Comparison of morbidity, 30-day mortality, and long-term survival after pneumonectomy and sleeve lobectomy for non-small cell lung carcinoma. Ann Thorac Surg 2005;79:968-73. [Crossref] [PubMed]
- Ma Z, Dong A, Fan J, et al. Does sleeve lobectomy concomitant with or without pulmonary artery reconstruction (double sleeve) have favorable results for non-small cell lung cancer compared with pneumonectomy? A meta-analysis. Eur J Cardiothorac Surg 2007;32:20-8. [Crossref] [PubMed]
- Naef AP, de Grüneck JS. Comparative results of right pneumonectomy and sleeve lobectomy. Cancer Chemother Rep 3 1973;4:97-9. [PubMed]
- Taghavi S, Birsan T, Pereszlenyi A, et al. Bilateral lung transplantation via two sequential anterolateral thoracotomies. Eur J Cardiothorac Surg 1999;15:658-62. [Crossref] [PubMed]
- Taghavi S, Birsan T, Seitelberger R, et al. Initial experience with two sequential anterolateral thoracotomies for bilateral lung transplantation. Ann Thorac Surg 1999;67:1440-3. [Crossref] [PubMed]
- Kirby TJ, Mack MJ, Landreneau RJ, et al. Initial experience with video-assisted thoracoscopic lobectomy. Ann Thorac Surg 1993;56:1248-52; discussion 1252-3. [Crossref] [PubMed]
- McKenna RJ Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6. [Crossref] [PubMed]
- Onaitis MW, Petersen RP, Balderson SS, et al. Thoracoscopic lobectomy is a safe and versatile procedure: experience with 500 consecutive patients. Ann Surg 2006;244:420-5. [PubMed]
- Swanson SJ, Herndon JE 2nd, D'Amico TA, et al. Video-assisted thoracic surgery lobectomy: report of CALGB 39802--a prospective, multi-institution feasibility study. J Clin Oncol 2007;25:4993-7. [Crossref] [PubMed]
- Bendixen M, Licht PB. Video-assisted thoracoscopic surgery versus open lobectomy for lung cancer: time for a randomized trial. Eur J Cardiothorac Surg 2017;52:398. [Crossref] [PubMed]
- Perna V, Carvajal AF, Torrecilla JA, et al. Uniportal video-assisted thoracoscopic lobectomy versus other video-assisted thoracoscopic lobectomy techniques: a randomized study. Eur J Cardiothorac Surg 2016;50:411-5. [Crossref] [PubMed]
- Perna V, Torrecilla JA, Carvajal AF, et al. Uniportal right upper video-assisted thoracoscopic surgery lobectomy: safe and feasible. J Vis Surg 2016;2:160. [Crossref] [PubMed]
- Ludwig C, Riedel R, Schnell J, et al. Inhalation with Tobramycin to improve healing of tracheobronchial reconstruction. Eur J Cardiothorac Surg 2009;35:797-800; discussion 800. [Crossref] [PubMed]
- Ludwig C, Stoelben E. A new classification of bronchial anastomosis after sleeve lobectomy. J Thorac Cardiovasc Surg 2012;144:808-12. [Crossref] [PubMed]
- Koryllos A, Stoelben E. Uniportal VATS intrapericardial left upper lobe bronchus-sleeve resection. Asvide 2018;5:032. Available online: http://asvidett.amegroups.com/article/view/22407
- Nakanishi K. Video-assisted thoracic surgery lobectomy with bronchoplasty for lung cancer: initial experience and techniques. Ann Thorac Surg 2007;84:191-5. [Crossref] [PubMed]
- Santambrogio L, Cioffi U, De Simone M, et al. Video-assisted sleeve lobectomy for mucoepidermoid carcinoma of the left lower lobar bronchus: a case report. Chest 2002;121:635-6. [Crossref] [PubMed]
- Mahtabifard A, Fuller CB, McKenna RJ Jr. Video-assisted thoracic surgery sleeve lobectomy: a case series. Ann Thorac Surg 2008;85:S729-32. [Crossref] [PubMed]
- Zhou S, Pei G, Han Y, et al. Sleeve lobectomy by video-assisted thoracic surgery versus thoracotomy for non-small cell lung cancer. J Cardiothorac Surg 2015;10:116. [Crossref] [PubMed]
- Huang J, Li S, Hao Z, et al. Complete video-assisted thoracoscopic surgery (VATS) bronchial sleeve lobectomy. J Thorac Dis 2016;8:553-74. [Crossref] [PubMed]
- Gonzalez-Rivas D, Delgado M, Fieira E, et al. Double sleeve uniportal video-assisted thoracoscopic lobectomy for non-small cell lung cancer. Ann Cardiothorac Surg 2014;3:E2. [PubMed]
- Gonzalez-Rivas D, Delgado M, Fieira E, et al. Left lower sleeve lobectomy by uniportal video-assisted thoracoscopic approach. Interact Cardiovasc Thorac Surg 2014;18:237-9. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fieira E, de la Torre M, et al. Bronchovascular right upper lobe reconstruction by uniportal video-assisted thoracoscopic surgery. J Thorac Dis 2014;6:861-3. [PubMed]
- Gonzalez-Rivas D, Fieira E, Delgado M, et al. Uniportal video-assisted thoracoscopic sleeve lobectomy and other complex resections. J Thorac Dis 2014;6:S674-81. [PubMed]
- Gonzalez-Rivas D, Fieira E, Delgado M, et al. Evolving from conventional video-assisted thoracoscopic lobectomy to uniportal: the story behind the evolution. J Thorac Dis 2014;6:S599-603. [PubMed]
- Gonzalez-Rivas D, Fieira E, Delgado M, et al. Is uniportal thoracoscopic surgery a feasible approach for advanced stages of non-small cell lung cancer? J Thorac Dis 2014;6:641-8. [PubMed]
- Huang J, Li J, Qiu Y, et al. Thoracoscopic double sleeve lobectomy in 13 patients: a series report from multi-centers. J Thorac Dis 2015;7:834-42. [PubMed]
- Ludwig C, Koryllos A, Zalepugas D, et al. Pneumologie 2015;69:403-8. [Review of the Literature for Interpretation of Endobronchial Wound Healing after Tracheobronchial Sleeve Resection]. [Crossref] [PubMed]
- Shao W, Phan K, Guo X, et al. Non-intubated complete thoracoscopic bronchial sleeve resection for central lung cancer. J Thorac Dis 2014;6:1485-8. [PubMed]
- Zhao Y, Chen H, Qiu T, et al. Robotic-assisted sleeve lobectomy for right upper lobe combining with middle lobe resection of lung cancer. J Vis Surg 2016;2:178. [Crossref] [PubMed]
Cite this article as: Koryllos A, Stoelben E. Uniportal video-assisted thoracoscopic surgery (VATS) sleeve resections for non-small cell lung cancer patients: an observational prospective study and technique analysis. J Vis Surg 2018;4:16.


