Video-assisted thoracoscopic surgery en bloc chest wall resection
Introduction
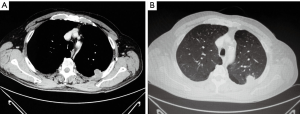
Since its first appearance on the stage of thoracic surgery in the 90s, video-assisted thoracoscopic surgery (VATS) provided a new standard approach for the treatment of early stage non-small cell lung cancer (NSCLC). Due to the more recent advancements in the technologies supporting the VATS techniques and in the experience of the surgeons, there has been a significant expansion in the application of this approach to more complex cases such as those of disease involving the chest wall, which occurs in approximately 5–8% of patients presenting with lung cancer. In this circumstance, the gold standard treatment is complete surgical resection via lobectomy and en bloc chest wall resection, which has a 40–50% 5-year survival when there is no lymph node involvement (Figure 1A,B) (1-4).
Thoracoscopic chest wall resection: gaining confidence
VATS application for this purpose was first described by Widmann et al. in 2000, who performed a complete thoracoscopic left upper lobe (LUL) wedge resection en bloc with two ribs for a T3 chest wall adenocarcinoma with no need of reconstruction of the defect, in a patient having undergone neoadjuvant radiotherapy (5). Since then, only a few cases have been reported in the literature. Between 2003 and 2010, Berry et al. tested a hybrid approach combining thoracoscopic technique to accomplish the pulmonary resection and a limited counter incision to perform the en bloc chest wall resection, avoiding scapular mobilisation and rib spreading (2). In the meanwhile, Demmy et al. followed three patients who underwent VATS lobectomy with en bloc chest wall resection and compared their outcomes with those of 14 additional patients who underwent thoracotomic approach for primary or secondary chest wall neoplasia (6).
In the wake of these experiences, some attempts were made for the resection of primary chest wall tumours, including chondrosarcoma and liposarcoma, barely considered so far due to their rare occurrence and the unknown effects on long-term results. Abicht et al. used a 3-incision approach to remove a mass involving the 6th rib and a piece of polytetrafluoroethylene (PTFE) to cover the defect (7). Hennon and Demmy performed an en bloc dissection of a chondrosarcoma involving the second and third ribs using a 3-portal approach and an additional incision to dissect the cartilaginous portion adjacent to the sternum. On postoperative day (POD) 43, the patient underwent a second operation using the same incisions to obtain negative margins, with no evidence of recurrence or lung herniation at 24 months from the initial surgery (8).
Finally, Gonzalez-Rivas updated the chest wall resection to the gaining recognition uniportal VATS technique; in the case report of a right upper lobe (RUL) adenocarcinoma was described the use of a single anterior incision to perform the lobectomy and to allow the thoracoscopic vision while resecting the fourth and fifth posterior ribs, which was achieved through a single posterior incision (for a total of two incisions) (9).
Techniques in comparison
The patient is positioned in lateral decubitus. It is widely agreed that limited en bloc chest wall resections can be accomplished by using port placement similar to that used for typical VATS anatomic resections, especially when the utility incision is placed close to the site of ribs excision. As regards the selection of the service port, the wider intercostal space justifies taking into account an anterior incision for easier access to hilar structures or extraction of the rib block (10). Demmy et al. performed the resection using two 12-mm access ports (a thoracoscopic port in the 8th or 9th intercostal space in the midaxillary line and an anterior 6th intercostal space incision) plus a 4-cm intercostal space access incision anterior to the involved ribs (6). Kawaguchi et al. dealt with a squamous cell carcinoma adherent to the dorsal edge of the chest wall, which required an additional 4th utility port along the paravertebral line to assist the dissection (11).
The thoracoscopic guidance allows a precise planning of the division of bone and soft tissue. A flexible or 30-degree angled camera allows to maximise visibility, and the vision can be achieved alternately through the camera port and the working ports. The hybrid procedure presented by Berry et al. consisted in a thoracoscopic lobectomy followed by the performance of a longitudinal paraspinal counter incision to insert standard tools under direct view or thoracoscopic guidance (2). The same principle was adopted by Gonzalez-Rivas, who applied it according to uniportal VATS technique. He performed a single 4-cm incision in the fifth intercostal space, followed by a single 4-cm posterior subscapular incision to resect the 4th and 5th posterior ribs under thoracoscopic guidance, for a total of two incisions. Gonzalez-Rivas also suggested that since most of the thoracoscopic rib resections reported are apical or posterior, a small lateral or posterior incision, according to the location of involvement, should be preferable for costal resection. Indeed, the presence of the scapula and the musculature of the back tend naturally to cover the defect, thus avoiding the necessity of a reconstruction, whereas if repair is needed the posterior approach allows an easy control from outside while placing the prosthesis and gives more confidence in achieving free oncologic margins, thus avoiding the performance of a third incision (9,12).
To divide bone near utility incision long-handled rib shears can be used, or alternately endoscopic osteotomes can pass through the port which has the most perpendicular angle with the rib. A high-speed drill burr can also be inserted in the utility incision, whereas Gigli saws can pass through a stab incision over the rib and endoscopic rongeurs can pass through the most available port (10). To achieve a complete thoracoscopic resection, Gonzalez-Rivas states that the endoscopic osteotome (also preferred by Demmy) and the rotary burr are the best options, albeit not indispensable (6,9). Abicht et al. agree that the rotating burr, due to its characteristics of fitting easily through a 5-mm port incision and providing quick and efficient rib transaction, is the tool of choice if available (7).
Chest wall tissues are divided by using unipolar cautery and cutting energy devices, which can also be used to cut the intercostal neurovascular bundles after positioning a right-angle clamp (10). Cerfolio et al. used the electrocautery to dissect intercostal muscles and vessels from inside through the chest retractor (13); Kawaguchi and others preferred the LigaSure instead, thus allegedly achieving a better hemostasis and avoiding unexpected neurologic sequelae due to the closeness of the cautery to the sympathetic chain and the intraspinal canal (11).
Hennon et al. described the resection of a left paramediastinal chondrosarcoma removed via laparoscopy that required an additional 5-mm left anterior incision: this allowed to dissect the cartilaginous portion of second and third ribs adjacent to sternum using the electrocautery and to control the internal thoracic artery with the LigaSure (8).
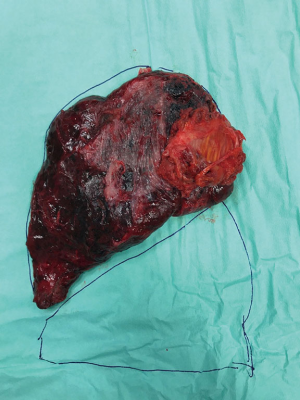
The rib-block is then removed thanks to endoscopic instruments capable of bending it away from the chest wall, for example, an endoscopic fan retractor, a lung grasper or a Diamond-Flex retractor. The specimen must be extracted using a wound protector or a nylon extraction sac (e.g., LapSac), taking care that it does not perforate the bag and spill tumour upon removal. The rib-block is oriented perpendicularly to the exit point and then extracted through the utility port or an alternative site, such as a subxiphoid approach (in that case the sternal lift technique allows to open subxiphoid space for easier extraction) (Figure 2) (6,10,13).
A structural chest wall support may be necessary for the event of loss of two or more ribs or defects >5 cm in diameter. Restoring rigidity and stability of the chest wall is important to avoid flail, scapular tip entrapment and lung herniation, as well as to seal the pleura and prevent infection. Abicht et al. described a reconstruction using a 2-mm thick patch of PTFE. The patch is measured based on extracted specimen and is sutured through utility incision (7). An additional reconstruction with titanium plates or absorbable products is described: Rocco et al. placed a vertical titanium plate under thoracoscopic guidance to avoid friction of the plate on the skin and to prevent the locking screws from puncturing underlying structures (lungs or pericardium) (4). Caruana et al. reported the resection of posterior arches of the right 6th to ninth ribs, 7th to 8th transverse processes and related musculature which required the reconstruction of the defect, carried out by using a polypropylene mesh reinforced with gentamicin-impregnated methyl methacrylate cement (3).
Results
The VATS technique for en bloc chest wall resection showed significant better postoperative progress, lower complication rates and shorter hospital stays without compromising the oncologic outcomes. In the pioneering case reported by Widmann, the remaining left lung (after LUL lobectomy) expanded with no evidence of air leak and the patient was discharged on the first POD (5). In the following study by Demmy et al. the patients having undergone thoracoscopic lobectomy and en bloc chest wall resection had no complications and were remarkably pain-free after 3 weeks. One out of three patients developed chronic pain syndrome that resolved by 18 months; the others two patients were not using pain medications and denied any significant discomfort. Two patients underwent chemotherapy on POD 58 and 42, and all the patients showed no evidence of disease at their last follow-up (respectively at 30, 20 and 10 months postoperatively) (6).
Berry referred that none of the patients treated with a planned hybrid approach needed conversion to thoracotomy. Resection details such as tumour size and some resected ribs were similar in this group and the group treated with the thoracotomic approach, and complete resection with negative margins was achieved in both. In the thoracotomy group, 40 out of 93 patients (43%) needed reconstruction with mesh, slightly more than the VATS hybrid group where the repair was performed in 4 out of 12 patients (33%). Nonetheless, the authors highlighted that patients in the thoracotomy group presented with larger tumours and hilar lymph node involvement (31% were higher than stage II), thus being less suitable candidates for the thoracoscopic approach (2).
As regards Hennon et al. study, 47 en bloc chest wall resections were performed: 17 (36%) by a VATS approach with no conversions and 30 (64%) by the standard open approach. The two groups were similar regarding baseline characteristics, except the VATS group having an older mean/median age (P=0.001). Thirty-one out of 47 cases were primary NSCLC mostly higher than stage IIB. Among these patients, the 90-day mortality was 26.7% in the VATS group and 25% in the thoracotomy group, probably on account of the patients in the first group being significantly older. Therefore, the hospital stay length and the necessity of reconstruction were significantly inferior in the VATS group. In conclusion, the stage matched survival curves for both approaches were superimposable (the difference between curves P=0.88) (14).
Discussion
Approximately 5% of lung resections for primary NSCLC involve en bloc chest wall resection for local invasion. Adjuvant preoperative radiotherapy, although still controversial, has been reported to favour excellent (>50%) survival rates, reduce local recurrence rates and limit the extent of resection in selected patients (5). Still, a traditional thoracotomy is associated with high levels of morbidity and mortality and involves a long and painful recovery.
Pain is believed to be caused mostly by stretching or retraction injury involving the intercostal nerve. Apparently, each nerve spared during thoracotomy enhance the postoperative pain, and even one less chest tube can help. This can be explained not only by nerve injury but rather by a “wind up” amplification of central nociception following extensive tissue trauma and pleural inflammation. By avoiding the rib spreading required by an open approach, postoperative pain is remarkably reduced, and pulmonary mechanics is better preserved, which is relevant since the first cause of mortality in combined chest wall and lung resection is respiratory insufficiency. There is particular evidence of these benefits in high-risk patients who might not tolerate a standard thoracotomy. The interior view provided by high-definition cameras allows a more accurate rib selection without destroying the integrity of chest wall and preserving the external musculature. The length of stays is significantly shortened since most of the patients are discharged on a POD 1 to 3 (2,5,6).
Disadvantages of the VATS technique may include the increased operative time due to higher technical difficulty, the smaller volume of the cases that limits the learning curve and the disfiguring result that might anyway occur for a rib resection. As raised by Hennon et al., the increased operative time also involves a prolonged anaesthesia with the related risks, more pronounced in the group of older and frail patients who represent the primary target of minimally invasive surgery (14). Nonetheless, Demmy et al. referred that operative times were gradually reduced while gaining more experience, and by the way occasionally prolonged operative times did not impair the outcomes (6).
The patients must be carefully selected based on the stage of the tumour, its size and the presence of hilar adenopathy, but also on the use of prior radiotherapy, which may limit hilar mobility, and the location of the chest wall involvement. Optimal candidates are patients whose chest wall invasion lies near to a favourable access for pulmonary resection, which requires a maximum of four ribs resected with no need of reconstruction. Thus, postscapular involvements are the best premise for successful VATS en bloc chest wall resection. Thoracoscopic resection of the first rib is especially difficult to perform due to the proximity of the thoracic outlet and the limited visualisation of the apex of the thorax. Therefore, tumours involving transverse processes or vertebral bodies are not suitable for VATS resection (1,2).
Concerning the amount of blood loss, it is acceptable, and the bleeding is also reduced thanks to the diminished use of FANS in patients medicated with an anticoagulant.
The possibility of dividing ribs via thoracoscopy may make superfluous the operative port incisions and opens the way for uniportal VATS: the camera is used in coordination with the instruments through a single incision, thus providing a vertical perspective on the target. The main disadvantage is the difficulty in achieving a proper coordination between the surgeon and the assistant (4,9).
Conclusions
VATS en bloc chest wall resection has been widely agreed to provide a safe and efficient alternative to the conventional thoracotomy for the resection of primary and secondary chest wall neoplasia, which is mostly represented by non-small cell lung cancer (NSCLC) with local invasion. Despite the absence of a randomized controlled study comparing the outcomes of the thoracoscopic approach with those of the open approach, patients treated with VATS technique presented a faster recovery and improved levels of activity, shorter chest tube duration and length of hospitalization, significantly less postoperative pain and reduced use of analgesics, reduced inflammatory response and lower rates of occurrence of chronic pain syndrome and others postoperative complications.
Although being initially addressed to non-complicated cases and small stage tumours, the thoracoscopic technique has been recently adopted for increasing complex cases such as patients requiring sleeve lobectomy and reconstruction or high-risk patients who might not tolerate a more demolition surgery. In patients presenting with weakened immune system due to an advanced age, comorbidity, aggressivity of the disease and neoadjuvant treatments the VATS en bloc is most recommended.
Enhancements in this direction are still required in terms of gaining technical proficiency, reducing the operative times and taking advantages of the latest innovations in order to extend the field of application of the VATS en bloc chest wall resection to more challenging cases, reducing the number of incisions, improving the visualization and reaching the optimal synergy between the surgeon and his assistant.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kara HV, Balderson SS, D'Amico TA. Challenging cases: thoracoscopic lobectomy with chest wall resection and sleeve lobectomy-Duke experience. J Thorac Dis 2014;6:S637-40. [PubMed]
- Berry MF, Onaitis MW, Tong BC, et al. Feasibility of hybrid thoracoscopic lobectomy and en-bloc chest wall resection. Eur J Cardiothorac Surg 2012;41:888-92. [Crossref] [PubMed]
- Caruana EJ, Solli P, Coonar AS. Hybrid video-assisted thoracoscopic surgery lobectomy and en-bloc chest wall resection for non-small cell lung cancer. J Thorac Dis 2016;8:E935-E937. [Crossref] [PubMed]
- Rocco G, Fazioli F, Martucci N, et al. Video-assisted thoracic surgery rib resection and reconstruction with titanium plate. Ann Thorac Surg 2011;92:744-5. [Crossref] [PubMed]
- Widmann MD, Caccavale RJ, Bocage JP, et al. Video-assisted thoracic surgery resection of chest wall en bloc for lung carcinoma. Ann Thorac Surg 2000;70:2138-40. [Crossref] [PubMed]
- Demmy TL, Nwogu CE, Yendamuri S. Thoracoscopic chest wall resection: what is its role? Ann Thorac Surg 2010;89:S2142-5. [Crossref] [PubMed]
- Abicht TO, de Hoyos AL. Chest wall resection and reconstruction: a true thoracoscopic approach. Innovations (Phila) 2011;6:399-402. [Crossref] [PubMed]
- Hennon MW, Demmy TL. Thoracoscopic resection and re-resection of an anterior chest wall chondrosarcoma. Innovations (Phila) 2012;7:445-7. [PubMed]
- Gonzalez-Rivas D, Fernandez R, Fieira E, et al. Single-incision thoracoscopic right upper lobectomy with chest wall resection by posterior approach. Innovations (Phila) 2013;8:70-2. [Crossref] [PubMed]
- Demmy TL, Yendamuri S, Hennon MW, et al. Thoracoscopic maneuvers for chest wall resection and reconstruction. J Thorac Cardiovasc Surg 2012;144:S52-7. [Crossref] [PubMed]
- Kawaguchi T, Tojo T, Kawai N, et al. A new minimally invasive technique of combined chest wall resection for lung cancer. Surg Today 2016;46:1348-51. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fieira E, Delgado M, et al. Uniportal video-assisted thoracoscopic sleeve lobectomy and other complex resections. J Thorac Dis 2014;6:S674-81. [PubMed]
- Cerfolio RJ, Bryant AS, Minnich DJ. Minimally invasive chest wall resection: sparing the overlying, uninvolved extrathoracic musculature of the chest. Ann Thorac Surg 2012;94:1744-7. [Crossref] [PubMed]
- Hennon MW, Dexter EU, Huang M, et al. Does Thoracoscopic Surgery Decrease the Morbidity of Combined Lung and Chest Wall Resection? Ann Thorac Surg 2015;99:1929-34; discussion 1934-5.
Cite this article as: Giaccone A, Solli P, Pardolesi A, Brandolini J, Bertolaccini L. Video-assisted thoracoscopic surgery en bloc chest wall resection. J Vis Surg 2017;3:73.


