Thoracoscopic wedge resection and segmentectomy for small-sized pulmonary nodules
Introduction
With advances in computed tomography (CT), the diagnosis of small-sized lung nodules and lung cancers with ground glass opacity (GGO) has increased. Based on the opinion by Ginsberg et al. that lobectomy is appropriate for lung cancer, the standard procedure has been lobectomy and lymph node dissection (1). However, small-sized lung nodules with GGO were not considered in their report. It is therefore unclear whether lobectomy is the appropriate procedure for small-sized lung nodules with GGO because limited resection may be sufficient. Noguchi et al. concurrently reported that wedge resection for small-sized, non-small cell lung cancers with GGO have been associated with good outcomes (2). Moreover, most nodules that are GGO-dominant have been shown to be adenocarcinoma in situ (AIS) or minimally invasive adenocarcinoma (MIA), which have good pathological prognoses (3,4). Therefore, the trend in surgery for small-sized GGO-dominant lung nodules has changed from lobectomy to limited resection. Limited resection generally refers to wedge resection or segmentectomy. Wedge resection has been widely performed for small-sized lung nodules to diagnose indeterminate lesions or cure small-sized GGO-dominant lung tumors, because the procedure is simple and easy (5). Although segmentectomy is generally thought to be more complex than wedge resection, the oncological outcomes of segmentectomy in a propensity-matched study were comparable to those of lobectomy for patients with early-stage non-small lung cell cancer (6). Segmentectomy has thus become widely used worldwide (7).
If limited resection is possible for a small-sized lung nodule, a thoracoscopic approach is a highly desirable, minimally invasive option (8,9). The thoracoscopic approach has better outcomes than thoracotomy with regard to quality of life and complications, and is preferred over thoracotomy for its advantages of decreased postoperative pain, shortened chest-tube duration, shortened length of hospital stay, faster return to preoperative activity levels, and preserved pulmonary function (8,9).
The combination of limited resection and minimally invasive surgery is, therefore, in great demand. In our institute, limited resection is preferred for small-sized GGO-dominant tumors. The aim of this article is to describe the role of limited thoracoscopic wedge resection and segmentectomy for small-sized lung nodules, with reference to recent literature. We will also describe our recent experience with thoracoscopic wedge resection and segmentectomy.
Indications for thoracoscopic wedge resection and segmentectomy
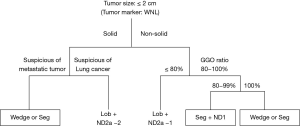
The prognosis of small-sized GGO-dominant lung cancers is generally good (3,4). Recently, Yano et al. reported that patients with these tumors were good candidates for limited wedge resection and segmentectomy (10). Important tumor characteristics in the indications for limited resection are tumor size and GGO ratio. Asamura et al. stated that tumors <2 cm in diameter with >75% GGO ratio on radiography were pathologically noninvasive (4). Nakata et al. indicated that patients with tumors with GGO ratios >50% should be considered candidates for limited resection, although those with a GGO ratio of 50% exhibited vessel infiltration and experienced local recurrence after wedge resection (11). Based on these reports, we determined that the indications for limited resection of indeterminate lung nodules should be tumor size <2 cm and a GGO ratio >80% to more strictly secure sufficient resection. Moreover, the choice between wedge resection and segmentectomy depends on tumor location and GGO ratio. Wedge resection was specifically planned for peripheral lung nodules with an anticipated easy curative resection of sufficient surgical margin and GGO ratio of almost 100%. On the other hand, segmentectomy was planned for lung nodules located in deep parenchyma with a GGO ratio of 80–100%, for which wedge resection was expected to be difficult in securing a sufficient surgical margin.
Thoracoscopic wedge resection and segmentectomy were also indicated for palliative cases in which heart and pulmonary function were poor because they are generally preferred to open lobectomy to reduce the effects of invasive procedures or to preserve pulmonary function.
Current issues and measures in the thoracoscopic wedge resection
Wedge resection for lung cancer is assumed to be more limited than segmentectomy for small-sized and peripheral lung tumors because it is difficult to secure an adequate surgical margin when the tumor location is deep to the visceral pleura. Mohiuddin et al. reported that margin distance in wedge resection affects local recurrence (12). Therefore, securing a sufficient surgical margin with wedge resection is very important.
Although wedge resection is simple and easy, precise resection presents unique challenges. For example, when the tumor is in the deep parenchyma, tumor identification is very difficult because almost all lung nodules with suspected cancer have GGO components, and cannot be palpated by the surgeon during thoracoscopy. Therefore, localization and precise resection of small-sized GGO lung tumors during thoracoscopic surgery is challenging (13-16). Although the most traditional method is CT-guided hookwire marking, serious complications, such as pneumothorax, hematoma, and air embolism, occasionally occur with this method (17,18). Therefore, to avoid these complications, the development of alternative methods has been a topic of discussion in recent years.
Gill et al. recently reported the usefulness of findings from a prospective clinical trial of image-guided video-assisted thoracoscopic surgery (iVATS), which creates percutaneous markings with two T-bars utilizing intraoperative C-arm CT (19). In this study, there were no intraoperative complications. Hence, it is expected that iVATS will become a major method of tumor identification in the future.
Technical characteristics and our improvements in thoracoscopic segmentectomy
Segmentectomy has some advantages compared to wedge resection. Segmentectomy is an anatomical resection, in which the targeted bronchus, pulmonary artery, and veins are anatomically divided. When a tumor is in the deep parenchyma, segmentectomy using three-dimensional (3D)-CT simulation enables the surgeon to correctly resect the tumor, secure the surgical margin, and pathologically evaluate metastasis to hilar lymph nodes. Furthermore, anatomical segmentectomy is preferred to lobectomy for small-sized lung tumors with GGO to preserve pulmonary function (20). The oncological outcomes of segmentectomy for peripheral small-sized lung cancer were not inferior to those of lobectomy (7).
Segmentectomy, however, is technically difficult, especially in thoracoscopic surgery. We therefore reported that 3D-CT simulation provides useful information for thoracoscopic surgery, serving to assist with the procedure and enable the performance of any segmentectomy or subsegmentectomy (21,22). Before the introduction of 3D-CT simulation, only simple segmentectomy was possible. 3D-CT simulation has enabled the performance of various types of thoracoscopic segmentectomy. Furthermore, we have improved thoracoscopic segmentectomy using a simplified technique. The most important technical process in anatomical segmentectomy is the division of the intersegmental plane. The visualization of an intersegmental plane is, therefore, a key process in segmentectomy. Accordingly, methods used to visualize the intersegmental plane (i.e., selective air supply using a bronchoscope, or injection of dye into the target segmental bronchus using a needle) have been reported (23,24). Although this process may be easy in open thoracotomy, it is considered difficult to perform in thoracoscopic surgery. To perform thoracoscopic segmentectomy, it is necessary to overcome the difficulty of creating the intersegmental plane. Therefore, we developed and evaluated the usefulness of a slip-knot method for creating an intersegmental plane during thoracoscopic segmentectomy (25). The essential device in this method is simply a slip-knot made from a monofilament suture, and the essential technique is simply pulling the slip-knot. Therefore, this method was simpler, easier, and of lower cost than any other conventional method.
Our original data of thoracoscopic wedge resection and segmentectomy based on our criteria for indications, and future prospects for wedge resection and segmentectomy
When we performed wedge resection, in September 2015, we introduced a hookwire method under general anesthesia in a hybrid operating room to avoid complications, including air embolism. We applied the hookwire method based on the assumption that air embolism might occur with spontaneous breathing but not under general anesthesia. We have used this method in 9 cases without any complications. Although the number of our cases is still small, we believe this method is useful for tumor identification in wedge resection (Figure 1).
In recent years, staplers and energy-based sealing devices have improved remarkably. In thoracoscopic surgery, these devices are useful in the limited working space of the thoracic cavity. The energy-based sealing devices have been widely used for various endoscopic surgeries, and have been particularly useful for the division of pulmonary vessels and the parenchyma along the intersegmental veins in thoracoscopic segmentectomy.
The slip-knot method and energy-based sealing technique simplify and reduce the difficulty of thoracoscopic segmentectomy. With the introduction of these methods, our thoracoscopic segmentectomy procedure has been fully developed since 2012 (Figure 2). Among 245 thoracoscopic segmentectomies performed since September 2004, we retrospectively examined surgical outcomes in 126 consecutive cases between March 2012 and December 2015, in which these methods were used. Since the introduction of these methods, surgical time, bleeding, and complications have been reduced.

Furthermore, we have been able to correctly resect non-visible or non-palpable tumors without use of any tumor markings with thoracoscopic segmentectomy. Wedge resection of these tumors has been thought to yield insufficient surgical margins because of their location in deeper parenchyma. Among 144 consecutive patients who underwent thoracoscopic segmentectomy between January 2012 and March 2016, we performed 58 thoracoscopic segmentectomies for non-visible or non-palpable tumors. While additional wedge resections followed segmentectomy to obtain sufficient surgical margins in 16 cases because initial margins were deemed insufficient, all tumors were accurately included in each specimen resected with planned segmentectomy. This extremely high accuracy rate can be attributed to pre- and intraoperative 3D-CT simulation. Thoracoscopic segmentectomy using 3D-CT simulation may be useful for small-sized lung nodules located in deeper parenchyma.
Recent reports demonstrated good outcomes for limited resection of small-sized lung cancers (10), especially in patients with GGO-dominant tumors. For thoracoscopic wedge resection and segmentectomy, the following patient selection criteria were used: (I) solid lung tumor <2 cm in diameter of indeterminate nature, but considered suspicious for metastatic lung tumor; (II) non-solid lung tumor, with planned resection of a cT1aN0M0 primary lung cancer, <2 cm in diameter, with a GGO ratio >80%, determined by high-resolution CT, in patients with good pulmonary function and who are able to tolerate lobectomy; (III) compromised resection in patients who are considered poor candidates for lobectomy because of limited cardiopulmonary reserve or other organ failure. Thoracoscopic surgery was indicated when we thought it could be used for limited resection. Our thoracoscopic surgical strategy for small-sized lung nodules is described in Figure 3. Consequently, we conducted approximately 20 wedge resections and 180 segmentectomies for lung cancer <2.0 cm and >80% GGO ratio since 2004. There were no recurrences with wedge resection and segmentectomy based on these criteria. After our segmentectomy procedure was fixed, using 3D-CT simulation, the slip-knot method to create the inflation-deflation line, and an energy-based sealing device during the division of pulmonary segmental vessels and parenchyma, our procedure was fully developed. The procedure was safe and familiar, although the result might depend on a learning-curve effect.
Thus, we have performed thoracoscopic wedge resection and segmentectomy for small-sized lung nodules using these methods, and the outcomes have been satisfactory as a curative operation. The choice of procedures for limited resection must be appropriately matched to each patient according to tumor size, GGO ratio, and tumor location. Limited, individualized resection will continue to evolve with applications such as CT and other new methods.
In conclusion, when indicated, thoracoscopic wedge resection and segmentectomy for small-sized lung nodules is appropriate, when used with the methods described herein.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22;discussion 622-3. [Crossref] [PubMed]
- Noguchi M, Morikawa A, Kawasaki M, et al. Small adenocarcinoma of the lung. Histologic characteristics and prognosis. Cancer 1995;75:2844-52. [Crossref] [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [Crossref] [PubMed]
- Asamura H, Hishida T, Suzuki K, et al. Radiographically determined noninvasive adenocarcinoma of the lung: Survival outcomes of Japan Clinical Oncology Group 0201. J Thorac Cardiovasc Surg 2013;146:24-30. [Crossref] [PubMed]
- Nakayama H, Yamada K, Saito H, et al. Sublobar resection for patients with peripheral small adenocarcinomas of the lung: surgical outcome is associated with features on computed tomographic imaging. Ann Thorac Surg 2007;84:1675-9. [Crossref] [PubMed]
- Hwang Y, Kang CH, Kim HS, et al. Comparison of thoracoscopic segmentectomy and thoracoscopic lobectomy on the patients with non-small cell lung cancer: a propensity score matching study. Eur J Cardiothorac Surg 2015;48:273-8. [Crossref] [PubMed]
- Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small lung cancer: a multicenter study. J Thorac Cardiovasc Surg 2006;132:769-75. [Crossref] [PubMed]
- Kaseda S, Aoki T, Hangai N, et al. Better pulmonary function and prognosis with video-assisted thoracic surgery than with thoracotomy. Ann Thorac Surg 2000;70:1644-6. [Crossref] [PubMed]
- Atkins BZ, Harpole DH Jr, Mangum JH, et al. Pulmonary segmentectomy by thoracotomy or thoracoscopy: Reduced hospital length of stay with a minimally-invasive approach. Ann Thorac Surg 2007;84:1107-12;discussion 1112-3. [Crossref] [PubMed]
- Yano M, Yoshida J, Koike T, et al. Survival of 1737 lobectomy-tolerable patients who underwent limited resection for cStage IA non-small-cell lung cancer. Eur J Cardiothorac Surg 2015;47:135-42. [Crossref] [PubMed]
- Nakata M, Sawada S, Yamashita M, et al. Objective radiologic analysis of ground-glass opacity aimed at curative limited resection for small peripheral non-small cell lung cancer. J Thorac Cardiovasc Surg 2005;129:1226-31. [Crossref] [PubMed]
- Mohiuddin K, Haneuse S, Sofer T, et al. Relationship between margin distance and local recurrence among patients undergoing wedge resection for small (≤2 cm) non-small cell lung cancer. J Thorac Cardiovasc Surg 2014;147:1169-75. [Crossref] [PubMed]
- Mack MJ, Gordon MJ, Postma TW, et al. Percutaneous localization of pulmonary nodules for thoracoscopic lung resection. Ann Thorac Surg 1992;53:1123-4. [Crossref] [PubMed]
- Bolton WD, Howe H 3rd, Stephenson JE. The utility of electromagnetic navigational bronchoscopy as a localization tool for robotic resection of small pulmonary nodules. Ann Thorac Surg 2014;98:471-5;discussion 475-6. [Crossref] [PubMed]
- Mack MJ, Shennib H, Landreneau RJ, et al. Techniques for localization of pulmonary nodules for thoracoscopic resection. J Thorac Cardiovasc Surg 1993;106:550-3. [PubMed]
- Ikeda K, Nomori H, Mori T, et al. Impalpable pulmonary nodules with ground-glass opacity: Success for marking pathologic sections with preoperative marking by lipiodol. Chest 2007;131:502-6. [Crossref] [PubMed]
- Ciriaco P, Negri G, Puglisi A, et al. Video-assisted thoracoscopic surgery for pulmonary nodules: rationale for preoperative computed tomography-guided hookwire localization. Eur J Cardiothorac Surg 2004;25:429-33. [Crossref] [PubMed]
- Horan TA, Pinheiro PM, Araujo LM, et al. Massive gas embolism during pulmonary nodule hook wire location. Ann Thorac Surg 2002;73:1647-9. [Crossref] [PubMed]
- Gill RR, Zheng Y, Barlow JS, et al. Image-guided video assisted thoracoscopic surgery (iVATS)-phase I-II clinical trial. J Surg Oncol 2015;112:18-25. [Crossref] [PubMed]
- Harada H, Okada M, Sakamoto T, et al. Functional advantage after radical segmentectomy versus lobectomy for lung cancer. Ann Thorac Surg 2005;80:2041-5. [Crossref] [PubMed]
- Oizumi H, Kanauchi N, Kato H, et al. Anatomical thoracoscopic pulmonary segmentectomy under three-dimensional multi-detector computed tomography simulation: a report of 52 consecutive cases. J Thorac Cardiovasc Surg 2011;141:678-82. [Crossref] [PubMed]
- Kato H, Oizumi H, Inoue T, et al. Port-access thoracoscopic anatomical subsegmentectomy. Interact Cardiovasc Thorac Surg 2013;16:824-9. [Crossref] [PubMed]
- Okada M, Mimura T, Ikegaki J, et al. A novel video-assisted anatomic segmentectomy technique: Selective segmental inflation via bronchofiberoptic jet followed by cautery cutting. J Thorac Cardiovasc Surg 2007;133:753-8. [Crossref] [PubMed]
- Zhang Z, Liao Y, Ai B, Liu C. Methylene blue staining: A new technique for identifying intersegmental planes in anatomic segmentectomy. Ann Thorac Surg 2015;99:238-42. [Crossref] [PubMed]
- Oizumi H, Kato H, Endoh M, et al. Slip knot bronchial ligation method for thoracoscopic lung segmentectomy. Ann Thorac Surg 2014;97:1456-8. [Crossref] [PubMed]
- Kato H, Oizumi H, Suzuki J, et al. Thoracoscopic anterior segmentectomy of right upper lobe using 3D-CT simulation and energy device. Asvide 2017;4:192. Available online: http://www.asvide.com/articles/1500
Cite this article as: Kato H, Oizumi H, Suzuki J, Hamada A, Watarai H, Nakahashi K, Sadahiro M. Thoracoscopic wedge resection and segmentectomy for small-sized pulmonary nodules. J Vis Surg 2017;3:66.


