Robotic resection of mediastinal masses: a decade of experience
Highlight box
Key findings
• Robotic-assisted resection of masses in all compartments of the mediastinum is associated with low conversion rate, high complete resection rate and excellent outcome.
What is known and what is new?
• Over the last 20 years, robotic-assisted thoracic surgery (RATS) has emerged as a promising technique for minimally invasive resection of mediastinal masses. Previous studies have shown its safety and feasibility for mediastinal tumor resection. However, long-term data including outcomes is rarely available.
• We report our experience over the past decade including high complete resection rate (partially after neoadjuvant treatment) and excellent outcome.
What is the implication, and what should change now?
• RATS is combining the advantages of video-assisted thoracic surgery while overcoming its limitations.
• With the rising availability of robotic platforms, great training capabilities and advantages like the three-dimensional view and dexterity due to articulating instruments, it is to be expected, that RATS will take a leading role in the treatment of mediastinal tumors.
Introduction
Due to their often asymptomatic presentation, mediastinal masses are most frequently discovered incidentally in chest radiographs or computed tomography (CT) scans. Symptoms occur only in about 60% of all patients (1). However, 85% of patients with malignant mediastinal tumors are symptomatic, while only 46% of patients with benign lesions display symptoms (2). Possible symptoms include cough, chest pain, dyspnea as well as dysphagia, Horner syndrome or paralysis of the limbs, vocal cords, diaphragm or systemic symptoms (3). As the different compartments of the mediastinum contain many vital structures and organs, the described symptoms are often caused by compression or invasion of the tumor into the respective surroundings. Systemic symptoms are often due to hormone, antibody or cytokine production (3).
Primary mediastinal tumors account for almost 50% of all mediastinal masses, and each compartment contains specific histopathologic entities that are more common than others (3). Approximately two thirds of all primary mediastinal tumors are benign (4).
Further diagnostics include CT scan, positron emission tomography (PET) scan and magnetic resonance imaging (MRI). Depending on tumor entity, biomarker screening can be an additional diagnostic tool, such as β-human chorionic gonadotropin (β-HCG) or alpha-fetoprotein (AFP) for mediastinal germ cell tumors (5).
The mediastinum has been compartmentalized and classified in various ways. A commonly used model, which we will follow in this review, is the division of the mediastinum into three compartments, the anterior, middle, and posterior mediastinum (6). In this three-compartment model, the superior mediastinum is combined with the anterior mediastinum to one combined anterior compartment (6).
Surgical therapy of mediastinal masses
Surgical removal is either the therapy of choice or an integral part of the multidisciplinary therapy for mediastinal tumors depending on tumor entity and stage of the disease (7). Complete resection is an important factor regarding the prognosis for almost all tumors mentioned, except for lymphomas and seminomas (7,8).
Due to the limited space, the complex anatomy and the variety of tumors within the mediastinum, an open approach has been the gold standard for mediastinal tumor surgery for a long time.
In the past 30 years, the utilization of video-assisted thoracic surgery (VATS) has increased, which led to a reduction in length of hospital stay (LOS), postoperative pain and overall morbidity (9). Although VATS shows these advantages, it also has limitations, such as two-dimensional view and the possible amplification of hand tremor caused by long endoscopic instruments (10). Additionally, VATS shows limited flexibility and range of instrument movement (11).
Robotic-assisted thoracic surgery (RATS) is now combining the advantages of VATS while overcoming its limitations. Due to the articulating instruments with improved ergonomics and tremor suppression, surgery in the narrow mediastinal space becomes more feasible, while the three-dimensional view allows for an easier identification of the vital structures to be dissected (12).
The first mediastinal tumor resection using RATS was a thymectomy performed in December 2000 and reported by Yoshino et al. in 2001 (13). Since then, thymectomy has become an increasingly popular robotic-assisted surgical intervention in thoracic surgery (14). As RATS allows for complex movements with good maneuverability in the narrow mediastinal space, RATS has also become increasingly popular for the treatment of other mediastinal tumors involving all mediastinal compartments (11,15-20). Moreover, due to the increasing availability of the da Vinci robotic platform, the numbers of robotic-assisted mediastinal surgeries performed are rapidly increasing (14).
Although past case series and retrospective studies have shown that RATS is feasible and safe, as of now, randomized studies comparing short-term and long-term outcomes between RATS, VATS and the open approach are missing. In this article, we review the different mediastinal tumors, their therapy, short-term as well as long-term outcomes and present our center’s data and experience over a decade regarding the treatment of mediastinal tumors using a robotic approach. We present this article in accordance with the TREND reporting checklist (available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-48/rc). (21).
Methods
We retrospectively analyzed all robotic-assisted resections of mediastinal masses performed at the Inselspital, Bern University Hospital, between September 2010 and October 2020 from our anonymized database.
Surgical technique (RATS)
The da Vinci robotic platform (Intuitive Surgical, Sunnyvale, CA) was utilized for all patients scheduled for robotic-assisted mediastinal tumor resection. As previously described by Zirafa et al. for tumors within the anterior compartment of the mediastinum, we favored an approach from the left side, apart from rare cases where the mediastinal tumor was located predominantly on the right side and therefore required a right sided approach (12). The patient was placed in a supine position with the affected side slightly elevated (usually left side). The ipsilateral arm was placed adjacent to the body and at lower position than the operating table to better expose the chest, maximize the working space and enable free movement of the robotic arms. A 12 mm camera port was inserted first into the fifth intercostal space in the anterior axillary line and followed by a diagnostic inspection of the chest cavity and the mediastinum. Afterwards, two 8 mm working ports were inserted, one in the fifth intercostal space in the midclavicular line and the second in the third intercostal space in the anterior axillary line. We insufflated carbon dioxide (CO2) up to a pressure of 8 to 10 mmHg. For instruments, a bipolar forceps was employed on the robotic arm positioned to the right of the camera trocar and a Cadiere forceps on the left, as previously described by Wei et al. (22).
Regarding masses in the posterior (and middle) mediastinum the patient was positioned in a lateral decubitus position with the ports placed in a linear fashion. Firstly, we placed the camera port in the midaxillary line and after a thoracoscopic overview we insufflated CO2 to further improve vision. Afterwards, a second port was inserted two intercostal spaces above in the anterior axillary line and the other port in the posterior axillary line in the same intercostal space as the camera port. Moreover, the selection of the respective intercostal spaces was dependent on the location of the tumor.
In cases where an infiltration of the lung was suspected preoperatively or during surgery, the concerning part of the lung was resected extra-anatomically en-bloc with the attached tumor. In cases of suspected infiltration of the pericardium, a partial localized pericardial resection was performed. Pericardial reconstruction is not routinely performed unless there is a risk of heart herniation, regardless of the anatomical location.
To allow an easier and safe retrieval of larger tumors, one of the incisions was increased and an endobag was used for specimen retrieval. A 20 or 24 French chest tube was placed through one of the ports. Criteria for chest tube removal was multifactorial, primarily guided by the volume of secretion, specifically a threshold of less than 200 mL secretion within a 24-hour period, and the absence of air leak. However, qualitative aspects of the fluid were also considered critical. The physical characteristics of the secretion, particularly its consistency and color, were evaluated.
For the different tumor entities, we applied the appropriate classifications in each case. For thymomas, we used the Masaoka-Koga staging system (23) and the histological type was determined using the WHO classification of thymomas (24). The clinical staging of thymic carcinoma was based on the TNM classification (25). Teratomas were classified based on the degree of differentiation into mature and immature.
Postoperative complications were recorded as such if they attained Grade II or higher according to the Clavien-Dindo classification of surgical complications (26).
Statistical analysis
Data is reported as means, standard deviation, ranges and percentages. Categorical variables were compared using two-tailed t-test and Chi-square test. Statistical calculations were made using SPSS IBM® (Version 29.0.0, www.ibm.com).
Ethics consideration
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical approval was not required as the research data contained no personal and sensitive data, including anonymized health-related personal data, which do not fall within the scope of the Federal Act on Research involving Human Beings (Swiss Federal Human Research Act, HRA). Written informed consent was obtained from all patients, and the study was approved by Thoracic Surgery Review Board Inselspital (approval number, TS03-2021/2; date of approval, 10 May 2021).
Results
Over a decade, 124 robotic-assisted resections of mediastinal masses were performed using the da Vinci robotic platform (Intuitive Surgical, Sunnyvale, CA, USA). From all patients, 67 were men and 57 were women. The median age was 53 years. Patient characteristics are summarized in Table 1.
Table 1
| Characteristics | Total | Mediastinal compartment | ||
|---|---|---|---|---|
| Anterior | Posterior | Middle | ||
| Age (years) | 51.22±18.02 | 51.52±18.29 | 46.31±15.59 | 71 |
| Sex | ||||
| Male | 67 (54.03) | 61 (54.46) | 5 (45.45) | 1 (100.00) |
| Female | 57 (45.97) | 51 (45.54) | 6 (54.55) | – |
Of 124 resected mediastinal masses, 112 masses were located in the anterior, 1 in the middle and 11 in the posterior mediastinum. A total of 33.06% (n=41) proved malignant, which were all localized in anterior compartment of the mediastinum (Table 2).
Table 2
| Tumor dignity | Total | Mediastinal compartment | ||
|---|---|---|---|---|
| Anterior | Posterior | Middle | ||
| Malignant | 41 (33.06) | 41 (36.61) | 0 | 0 |
| Benign | 83 (66.94) | 71 (63.39) | 11 (100.00) | 1 (100.00) |
In 112 cases the mass was localized in the anterior mediastinum. Histopathological tumor entities in the anterior mediastinum are shown in Table 3. Malignancy occurred in 41 patients (36.61%), and in 71 patients (63.39%) a benign neoplasm was found. Out of all malignant tumors, 37 (90.24%) were thymomas, 3 (7.32%) were metastases of other cancer and 1 (2.44%) was a thymic carcinoma.
Table 3
| Histopathological findings | Anterior mediastinal compartment (n=112) |
|---|---|
| Tumor size (cm) | 5.16±3.41 |
| Histology | |
| Bronchogenic cyst | 1 (0.89) |
| Chondroma | 1 (0.89) |
| Goiter | 1 (0.89) |
| Granulomatous infiltration of the thymus (sarcoidosis) | 1 (0.89) |
| Hamartoma | 1 (0.89) |
| Lipoma | 2 (1.79) |
| Mesothelial cyst | 3 (2.68) |
| Metastasis | 3 (2.68) |
| Solitary fibrous tumor | 1 (0.89) |
| Teratoma | 3 (2.68) |
| Thymic atrophy | 2 (1.79) |
| Thymic carcinoma | 1 (0.89) |
| Thymic cyst | 11 (9.82) |
| Thymic hyperplasia | 34 (30.36) |
| Thymic hyperplasia and thymitis | 1 (0.89) |
| Thymitis | 3 (2.68) |
| Thymolipoma | 5 (4.46) |
| Thymoma | 37 (33.04) |
| Thyroid hyperplasia | 1 (0.89) |
| Resection margin | |
| R0 | 109 (97.32) |
| R1 | 2 (1.79) |
| R2 | 1 (0.89) |
In 11 out of the 124 cases, the mediastinal mass was located in the posterior mediastinum. All of these masses have been classified as benign after final histopathological evaluation (Table 2). Histopathological tumor entities in the posterior mediastinum are shown in Table 4.
Table 4
| Histopathological findings | Posterior mediastinal compartment (n=11) |
|---|---|
| Tumor size (cm) | 5.08±2.88 |
| Histology | |
| Castleman disease | 1 (9.09) |
| Esophageal duplication cyst | 1 (9.09) |
| Ganglioneuroma | 2 (18.18) |
| Neurofibroma | 1 (9.09) |
| Schwannoma | 5 (45.45) |
| Thoracic duct cyst | 1 (9.09) |
| Resection margin | |
| R0 | 10 (90.91) |
| R1 | 1 (9.09) |
| R2 | 0 |
The sole case of a mass in the middle mediastinum was a pericardial cyst (4.7 cm in size) close to the right auricle of the heart.
Histopathology showed that the mean size of the resected mediastinal masses in the anterior compartment was 5.16±3.41 cm (Table 3) (range, 0.5 up to 21.5 cm). Mean size for resected masses in the posterior mediastinum was 5.08±2.88 cm (Table 4) with the largest tumor resected being 8.7 cm in diameter (range, 2.2 up to 8.7 cm).
Complete resection was achieved in 96.77% (n=120) with a complete resection rate of 97.32% (n=109) for anterior, 90.91% (n=10) for posterior and 100.00% (n=1) for middle mediastinal masses (Tables 3,4). Overall conversion rate was 0.81% (n=1) (Table 5). In this case, a manubriotomy was performed for extended resection due to local tumor invasion into the brachiocephalic vein.
Table 5
| Outcomes | Total (n=124) | Mediastinal compartment | P value (anterior vs. posterior) | ||
|---|---|---|---|---|---|
| Anterior (n=112) | Posterior (n=11) | Middle (n=1) | |||
| Chest tube removal (POD) | 2.05±2.49 | 1.86±1.92 | 4.09±5.54 | 1 | 0.21 |
| LOS | 3.45±2.95 | 3.28±2.37 | 5.18±6.46 | 4 | 0.35 |
| Conversion | 1 (0.81) | 1 (0.89) | 0 (0.00) | 0 (0.00) | 0.75 |
| Patients with postoperative complications (within 30 days) | 16 (12.90) | 15 (13.39) | 1 (9.09) | 0 (0.00) | 0.68 |
POD, postoperative day; LOS, length of hospital stay.
Ten tumors arising from the anterior mediastinum (8.93%) received neoadjuvant therapy, of those, 8 cases were thymomas, one case was a teratoma and one case a metastasis of non-small cell lung cancer.
Mean LOS was 3.45±2.95 days for all robotic-assisted mediastinal mass resections with a mean LOS of 3.28±2.37 days for anterior and 5.18±6.46 days for posterior mediastinal masses. One thymectomy was performed in an outpatient setting.
Regarding conversion rate (P=0.75), chest tube duration (P=0.21), LOS (P=0.35) and postoperative complications within 30 days after surgery (P=0.68), no statistical significance could be found between the anterior and posterior compartment using Chi-square tests (conversion rate and postoperative complications) and two-tailed t-tests (chest tube duration and LOS) (Table 5).
Sixteen patients showed postoperative complications (12.90%) out of all mediastinal mass resections. Postoperative complications are listed in Table 6. Two patients showed multiple complications. One patient in the posterior mediastinal mass group (thoracic duct cyst) presented with postoperative chylothorax, atrial fibrillation and pneumonia. One patient in the anterior mediastinal mass group (thymoma) presented with postoperative pneumonia, delirium and myasthenic crisis, requiring reintubation and tracheostomy due to respiratory failure. Two patients in the anterior mediastinal tumor group showed slight worsening of myasthenic symptoms, treated by medication only. In three cases reoperation was required. Indication for reoperation was postoperative bleeding (n=1), chylothorax (n=1) and chest wall hernia (n=1). Another case of postoperative chylothorax was successfully treated conservatively with fasting and parenteral nutrition over 1 week. Postoperative 30- and 90-day mortality rate was 0.00% for all mediastinal mass resections.
Table 6
| Complications | Total | Mediastinal compartment | ||
|---|---|---|---|---|
| Anterior | Posterior | Middle | ||
| Mediastinal mass resections | 124 | 112 | 11 | 1 |
| Patients with complications | 16 (12.90) | 15 (13.39) | 1 (9.09) | 0 (0.00) |
| Postoperative complications | ||||
| Anaemia | 1 (0.81) | 1 (0.89) | – | – |
| Atrial fibrillation | 2 (1.61) | 1 (0.89) | 1 (9.09) | – |
| Bleeding | 1 (0.81) | 1 (0.89) | – | – |
| Chest wall hernia | 1 (0.81) | 1 (0.89) | – | – |
| Chylothorax | 2 (1.61) | 1 (0.89) | 1 (9.09) | – |
| Delirium | 2 (1.61) | 2 (1.79) | – | – |
| Empyema | 1 (0.81) | 1 (0.89) | – | – |
| Hypertensive derailment | 1 (0.81) | 1 (0.89) | – | – |
| Myasthenic crisis | 1 (0.81) | 1 (0.89) | – | – |
| Pneumonia | 5 (4.03) | 4 (3.57) | 1 (9.09) | – |
| Urinary tract infection | 1 (0.81) | 1 (0.89) | – | – |
| Worsening of myasthenic symptoms | 2 (1.61) | 2 (1.79) | – | – |
| None | 108 (87.10) | 97 (86.61) | 10 (90.91) | 1 (100.00) |
Mean follow-up for patients with thymoma, thymic carcinoma, teratoma and metastasis of other cancer was 3.33±2.88 years or 40.00±34.55 months (Table 7). Out of these 44 patients, 5-year follow-up was reached in 15 (34.09%) cases. In these patients (n=15) 5-year overall survival (OS) was 100% and 5-year recurrence-free survival (RFS) was 86.67%, with three patients showing recurrent disease. One patient with thymoma developed recurrence within 3.51 years and the only patient with thymic carcinoma showed early recurrent disease after 1.18 years. One of three patients with teratoma also showed recurrence within 4 years.
Table 7
| Follow-up | Total (n=44) | Histology | |||
|---|---|---|---|---|---|
| Thymoma (n=37) | Thymic carcinoma (n=1) | Teratoma (n=3) | Metastasis of other cancer (n=3) | ||
| Follow-up (years) | 3.33±2.88 | 3.40±2.98 | 6.2 | 3.13±1.77 | 1.75±2.58 |
| 5-year follow-up | 15 (34.09) | 14 (37.84) | 1 (100.00) | – | – |
| 5-year OS | 15 (100.00) | 14 (100.00) | 1 (100.00) | – | – |
| 5-year RFS | 13 (86.67) | 13 (92.86) | 0 (0.00) | – | – |
| Recurrence | 3 (6.82) | 1 (2.70) | 1 (100.00) | 1 (33.33) | – |
| Time until recurrence (years) | 2.35±1.65 | 3.51 | 1.18 | N/A | – |
OS, overall survival; RFS, recurrence-free survival; N/A, not available.
Discussion
Data collected over a decade at our center shows that RATS for mediastinal mass resection and all its various entities is a feasible and safe approach with low conversion rates, high complete resection rates and excellent postoperative outcomes. These results are also supported by the findings of previous case series (10,14,16,20).
In the past, complete and safe resections using RATS were reported by several authors, for tumors up to 9.5 cm in size and for tumors infiltrating the surrounding structures like the pericardium, lung or phrenic nerve (27-29). Our data shows that mediastinal masses up to 21.5 cm can be resected using a minimally invasive robotic approach. The final histopathologic result of the resected mediastinal mass with a size of 21.5 cm showed a large thymic hyperplasia. Therefore, as some of these mediastinal masses are soft, tumor size should not be considered as an absolute contraindication for a minimally invasive approach through RATS, especially as a subxiphoid port could allow for retrieval of large specimens.
In a decade, only one case of locally advanced thymoma required a conversion to an open approach due to local tumor invasion into the surrounding structures, requiring a manubriotomy and partial resection of the brachiocephalic vein.
Malignant mediastinal tumors
The malignancy rate of 33.06% corresponds to the expected rate previously published by Strollo et al., who found that approximately two thirds of all mediastinal tumors are benign and one third shows malignancy (4).
The rate of 33.93% (n=38) for thymic malignancies in the anterior mediastinum in our cohort (thymoma and thymic carcinoma) correlates with the previously reported rate of approximately 35% for thymic malignancies in anterior mediastinal masses published by Carter et al. (5). With a mean age of 51.22±18.02 years in our cohort (Table 1), the overall rate for thymomas in the anterior mediastinum was 33.04% (Table 3). In our cohort of 112 patients with anterior mediastinal masses, 81 patients were aged above 40 years. Among this subgroup, the incidence of thymomas was 41.98% (n=34), which closely aligns with the anticipated prevalence of approximately 50%, as reported by Carter et al. for patients above the age of 40 years (5). In accordance to this, the rate of benign thymic lesions is distinctly higher in our cohort. This discrepancy may arise from the practice at our institution, where biopsies are not routinely conducted to mitigate the risk of tumor cell dissemination. Studies have shown, that fine needle aspiration of mediastinal tumors can lead to misdiagnosis in 6.8% of cases (30). In accordance with the current ESMO clinical practice guidelines (31), we usually perform an upfront diagnostic and therapeutic resection of the suspicious lesion after complete neurological (evaluation of myasthenia gravis), radiological and laboratory work-up. In case of suspected thymic carcinoma and depending on tumor stage, a multimodality approach with neoadjuvant chemotherapy can be recommended. Upfront diagnostic and therapeutic resection might lead to the higher percentage of benign thymic lesions found within histopathological results (Tables 2,3).
Anterior compartment
Half of all mediastinal masses are located in the anterior compartment, and 59% of those are malignant, while only 29% of masses in the middle and 16% of masses in the posterior compartment show malignancy (2). The most common tumor entities in the anterior compartment are thymomas, thyroid neoplasms, teratomas and lymphomas (3).
Thymomas
Thymomas account for 20% of all masses in the anterior mediastinum in adults and have an incidence of 0.15 cases per 100,000 (3). While being uncommon in children, the incidence increases substantially with age. According to Carter et al., thymomas make up to 50% of all masses in the anterior mediastinum above the age of 40 years, without difference in gender distribution (5). Most often, thymomas are an incidental finding, since only a third of the patients display symptoms related to local tumor compression or invasion (3). Parathymic syndromes such as myasthenia gravis, hypogammaglobulinemia and red cell aplasia can occur in association with thymomas. Myasthenia gravis is the most common parathymic syndrome and is more frequent in women. It is associated with 30–50% of all thymomas (3).
The clinical staging is based on the type of thymoma, tumor stage and degree of invasion into the surrounding structures. The Masaoka stage and WHO histological classification are independent prognostic factors of thymoma after initial complete resection in regards of disease-free survival (DFS) and OS (32,33).
Other thymic malignancies include thymic carcinomas and thymic carcinoids (3). Benign and common thymic lesions include thymic hyperplasias, thymic cysts and thymolipomas (1,3).
Since locally advanced thymomas are at greater risk for incomplete resection, neoadjuvant chemotherapy has been used as an attempt to improve complete resection rates, particularly for extended tumors (34-36). However, currently, there exists no consensus regarding its advantage, necessitating randomized trials to elucidate its role in shaping future treatment strategies. In our cohort 21.62% (n=8) of patients with thymoma (n=37) received neoadjuvant therapy and in conjunction with the high complete resection rate, this indicates, that neoadjuvant treatment followed by robotic-assisted resection could be a feasible option for future multimodality treatment regimens for advanced thymomas compared to the standard open transsternal approach.
Yuan et al. reported a disease recurrence rate for thymomas of 16.9 % and 5-year as well as 10-year DFS rates of 84.0% and 73.0%, respectively. They also reported 5- and 10-year OS rates of 91.0% and 74.0%, respectively, for patients with thymomas, who underwent complete surgical resection (open or minimally invasive) and did not receive perioperative chemotherapy or radiation (33). In our study, the 5-year RFS rate (92.86%) and 5-year OS rate (100.00%) for thymomas (Table 7) are slightly higher. Yuan et al. found that the Masaoka stage and WHO classification are independent predictors of DFS and OS (33). Unfortunately our anonymized data set did not include this information, hence, comparison of these rates is very limited. Other explanations for the difference in rates, apart from tumor staging, could be on the one hand that in our cohort currently only 37.84% of patients with thymoma have reached the 5-year follow-up (Table 7) or on the other hand that 21.62% of patients with thymoma in our cohort received neoadjuvant therapy, which was an exclusion criterion in the study presented by Yuan et al. (33).
Germ cell tumors
Germ cell tumors make up for 15–20% of all masses in the anterior mediastinum with the most common subtype being teratoma (3,5). Teratomas are more likely to be found in women and younger patients under 20 years of age (5). Most teratomas are mature and contain fully developed tissue. These mature teratomas are benign and therefore, show a very good prognosis after complete resection (37). However, while being rare, mediastinal teratomas can also be malignant and contain immature tissue with poorer prognosis (38).
Other germ cell tumors that can be found in the anterior mediastinum include malignant seminomas and other malignant non-seminomatous germ cell tumors, which are more common in men (3). Mediastinal seminomas show a 5-year OS of over 90% up to 100% and are primarily treated with chemotherapy eventually followed by radiation or surgery (39,40), while non-seminomatous germ cell tumors only have a 5-year OS of 40–50% after treatment, such as chemotherapy and surgery (41).
Mediastinal teratoma
In 2.68% of all resected anterior mediastinal masses (Table 3) and in 1.23% of patients above the age of 40 years teratoma was found. This is coherent with the expected rate of under 5% for teratoma in anterior mediastinal masses in patients above the age of 40 years published by Carter et al. (5). Although histopathology revealed no degree of cellular immaturity in all three cases of resected teratoma, one case of these mature teratomas received neoadjuvant therapy and showed local recurrence within 4 years. Unfortunately, our anonymized data set does not include further information about this case, like e.g., previous histopathological results from potential biopsies or reasons that led to this treatment plan.
Thyroid and parathyroid neoplasms
Tumors that arise from the thyroid gland or substernal goiters, which extend from a thyroid lobe into the mediastinum, can present as mediastinal masses. According to Carter et al., they can make up for 10–30% of all masses in the anterior mediastinum and their incidence increases with age (5). Another tumor entity that can be found within the anterior compartment of the mediastinum is the parathyroid adenoma, which can lead to hyperparathyroidism (3).
In our cohort only 1.78% of cases (n=2) have been thyroid lesions. In comparison to an expected rate between 10–30% for substernal goiters (5), this is probably due to the fact, that substernal goiters at our center are resected through a cervical approach with potential manubriotomy or sternotomy without using a transpleural robotic approach.
Mediastinal cysts
Thymic cysts were found in eleven cases. All other cystic mediastinal lesions found in our cohort comprise 5.65% (n=7) of all mediastinal masses, which is lower than the expected rate of 12–20% described by Duwe et al. (3). This might be the result of an overall low prevalence of these tumor entities and the sample size of our cohort. In 2.42% (n=3) of all mediastinal lesions histopathology revealed mesothelial cysts, which is slightly lower than the anticipated rate of 3–6% reported by Bouma et al. (42). While the most common location for bronchogenic cysts as well as pericardial cysts within the mediastinum is the middle compartment (3,43), the singular bronchogenic cysts in our study was located in the anterior mediastinal compartment.
Lymphomas
Mediastinal lymphomas are generally considered the second most common primary tumors within the anterior compartment of the mediastinum and account for 25% of all anterior mediastinal masses, with a higher prevalence in younger patients (5). While commonly involving the mediastinum, only a small percentage of lymphomas primarily arise from mediastinal structures (5). If lymphoma is suspected based on the preliminary diagnostic, surgical removal is not the therapy of choice. However, surgical biopsy can be considered.
Others
Furthermore, lipomas, lymphangiomas and aortic aneurysms are described as potential differential diagnosis for mediastinal masses (3,44).
Middle compartment
The middle mediastinum contains mainly congenital cysts, which comprise of 12–20% of all mediastinal masses. Foregut cysts are the prevailing type of cysts, with bronchogenic cysts and enterogenous cysts constituting the most frequent subtypes (3). Pericardial cysts fall within the category of mesothelial cysts (3). Additionally, lymphangiomas, as mentioned above, also exist in the middle mediastinum (3).
In our cohort, the solitary mass in the middle compartment of the mediastinum was identified as a pericardial cyst, measuring 4.7 cm and located in close proximity to the right auricle of the heart.
Posterior compartment
In the posterior mediastinum, neurogenic tumors are the most common tumor entity, which are further differentiated based on cell type, such as nerve sheath tumors and tumors arising from autonomic ganglia. Nerve sheath tumors account for 40–65% of all neurogenic mediastinal masses with the most common ones being benign schwannomas (3,45). Malignant nerve sheath tumors include malignant neurofibromas, malignant schwannomas and neurogenic fibrosarcomas (3). Tumors arising from autonomic ganglia include benign ganglioneuromas as well as malignant ganglioneuroblastomas and neuroblastomas, which are predominantly occurring in young children (3).
Neurogenic tumors in the mediastinum
In our cohort 72.73% (n=8) of masses within the posterior mediastinum were intrathoracic neurogenic tumors, such as schwannomas, ganglioneuromas and one neurofibroma. Although approximately 70–80% of neurogenic tumors are benign and often an asymptomatic incidental finding, due to their continuous growth, these tumors can become symptomatic over time and have the potential to become malignant (45). Therefore, surgical removal, if feasible, is the treatment of choice (46). In their systematic review, Straughan et al. have demonstrated, that RATS appears to be superior compared to open approaches for resecting mediastinal tumors, including those within the posterior compartment. Furthermore, patient outcomes after RATS are comparable to those achieved with VATS (11). In a study conducted by Li et al. comparing the clinical efficacy of RATS for resection of posterior mediastinal neurogenic tumors in comparison to VATS, they found a significant lower estimated blood loss and shorter LOS in the RATS group (17).
Other mediastinal masses
Furthermore, other histopathologic entities were found in the different compartments of the mediastinum, such as chondromas, hamartomas and solitary fibrous tumors. All of these entities are very rare, hence, the literature is reduced to case reports or small case series. Chondromas are benign tumors of hyaline cartilage and present as solid, homogeneous, well-demarcated lesions in the CT scan (47). Hamartomas are most typically located in the lung, however, they can also be found in various other parts of the body and have only been reported within the mediastinum in few cases. Since they are generally asymptomatic and considered to be benign tumors, they are usually incidental findings (48). Solitary fibrous tumors (SFTs) are a spectrum of rare mesenchymal tumors, that are predominantly asymptomatic and benign with a low rate of recurrence (8%), however, approximately 10–20% are locally aggressive or malignant and can exhibit a high rate of recurrence (63%), even after complete resection (49). Therefore, although there are no official guidelines yet, a regular follow up regime, especially for more aggressive tumors, is advisable (49). For the singular, benign SFT found in our cohort, no regular follow-up was performed.
Another rare finding was one case of Castleman disease, which describes a group of at least four disorders, which share histopathological features but have different etiology, presentation, treatment and outcome (50). The benign unicentric Castleman disease can, like in our case, present as a mediastinal mass and surgery is the therapy of choice (50).
Postoperative outcomes
Chest tube duration, LOS and complete resection rates
Li et al. reported that the median day of chest tube removal was 3 days and median postoperative length of stay was 4 days in 106 cases of RATS mediastinal mass resection (51). Similar results have been published by Kneuertz et al. with mean duration of pleural drainage of 2±1.1 days and a median length of stay of 3 days for 20 patients undergoing RATS thymectomy (27). Our data shows similar results with chest tube removal on mean postoperative day 2.05±2.49 (median: 1 day) for all RATS mediastinal mass resections and 1.86±1.92 (median: 1 day) for anterior mass resections. LOS in our cohort was also slightly shorter with 3.45±2.95 days for all RATS mediastinal mass resections (median: 3 days) compared to the 4 days reported by Li et al. (51).
Complete resection rate for all thymic neoplasms in our study was 98.95% and complete resection rate for thymomas was 97.30%, which is higher compared to the complete resection rate of 90% for RATS thymectomies published by Kneuertz et al. (27) and similar to the rate of 97.9% for VATS thymectomies reported by Agatsuma et al. (52). Burt et al. presented in their retrospective analysis of thymectomies registered within the ITMIG database complete resection rates of 96% for open and minimal invasive approaches after a propensity matched analyses of both groups (53). Overall, our data is coherent with previous findings, and shows, that high rates of complete resection for mediastinal masses are achievable and not compromised by a minimally invasive approach, especially RATS.
Postoperative complications
The rate of postoperative complications (12.90%) for all performed RATS mediastinal mass resections in our cohort (n=124) is similar to the previously published rate by Kneuertz et al. with 15% of postoperative complications Grade II or higher (27) according to the Clavien-Dindo Classification (26) and higher than the rate of 5.7% for overall postoperative complications after RATS mediastinal mass resections published by Li et al. (51). The postoperative complications that occurred in our cohort (Table 6) correspond to known possible postoperative complications after thoracic surgery.
RATS versus VATS
Regarding the current status of RATS as a surgical approach for the resection of mediastinal masses, a systematic review published by O’Sullivan et al. has shown that minimally invasive thymectomies performed by RATS and VATS are associated with less postoperative complications, reduced blood loss, shorter LOS and a lower positive margin rate compared to open techniques, while no significant difference regarding these parameters could be found between RATS and VATS (9). This is coherent with the findings in the systematic review and meta-analysis of Fok et al., who found no statistical difference in conversion rates, LOS or postoperative pneumonia for thymectomies performed through RATS or VATS (54). Comparing the minimally invasive techniques for mediastinal mass resections, Alvarado et al. showed in their analysis of the National Cancer Database, that RATS was associated with a significantly lower conversion rate, shorter LOS and fewer positive pathologic margins (55). Li et al. reported that RATS in comparison to VATS was associated with a significant reduction of the incidence of overall postoperative complications and a shorter LOS for overweight and obese patients (51). However, they found no significant differences regarding postoperative duration of pleural drainage, postoperative complications of Grade III according to Clavien-Dindo or higher, or in-hospital mortality rate. In comparison to that, in a systematic review and meta-analysis conducted by Shen et al., RATS was associated with fewer postoperative pleural drainage days, shorter LOS and fewer postoperative complications in comparison to VATS (56).
In summary, there is no consensus on minimally invasive techniques in the current literature. To date, there are no randomized, controlled trials comparing resection of mediastinal masses by RATS and VATS, which makes evidence-based decision-making regarding the ideal therapeutic approach difficult. However, current data indicates that thymectomies performed by RATS are at least comparable to VATS (9,54) and RATS might have some advantages over VATS (51,55,56) in regards of perioperative outcomes. To further assess oncological outcomes, long-term follow-up data is required.
Randomized controlled trials comparing RATS and open approaches for the resection of mediastinal masses are difficult to execute, due to the vast difference in invasiveness of the compared approaches. Since current data suggest that a minimally invasive approach is superior to open surgery (9,11), a minimally invasive approach should be the preferred surgical approach for mediastinal masses. However, an open approach can be beneficial and necessary in larger and locally advanced or extended tumors infiltrating the surrounding tissues.
Our single center experience
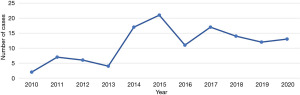
Following the inaugural robotic-assisted resection of a mediastinal mass utilizing the da Vinci robotic system at our institution, we observed a progressive increase in its application (Figure 1). RATS has subsequently emerged as the favored approach for mediastinal tumor resections, particularly within the anterior mediastinum. As our proficiency with the da Vinci system expanded over time, we noted a corresponding decrease in duration of operation (Figure 2).
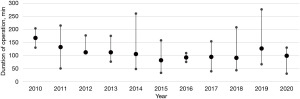
Initial pain management strategies predominantly involved the use of epidural catheters. However, this was later supplanted by intraoperative intercostal blocks, leading to reductions in both LOS and chest tube duration.
It is crucial to underscore that the limited number of cases per annum implies that patients with complications, and thus outliers, exert a substantial influence on the mean LOS per year, as delineated in Figure 3. This observation also extends to the mean chest tube duration, which is similarly susceptible to distortion by outliers or patients with complications (Figure 4).
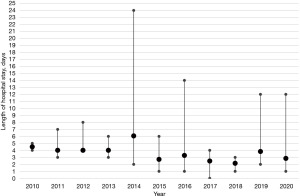
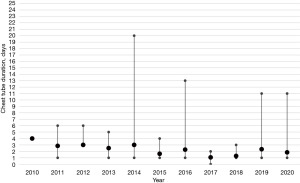
Overall, the data presented herein provides an account of our experiences with robotic-assisted resections of mediastinal masses. The observed trends suggest a potential benefit of this approach, particularly in terms of operative duration and patient recovery times. However, it is important to note that these findings are based on a limited number of cases and further studies are needed to confirm these results. The impact of outliers on the mean length of stay and the mean chest tube duration underscores the need for careful interpretation of the data. While our findings lend some support to the use of the robotic approach, we acknowledge the need for continued evaluation and refinement of these techniques in the pursuit of optimal patient outcomes.
Moreover, it is essential to recognize the intricacies of our country’s healthcare system, which operates on the basis of diagnosis-related groups (DRGs). These DRGs serve as a framework for categorizing patients based on their diagnoses and treatments. Patients with the abovementioned mediastinal masses undergoing specific therapies are generally expected to have an absolute minimum hospital stay of 2 to 3 days. Deviation from this expectation may lead to penalty fees or reduced reimbursement. While the primary determinants of LOS are multifactorial, including clinical severity, treatment protocols, and patient-specific factors, the DRG system naturally exerts some influence. It impacts resource allocation, discharge planning, and financial considerations within the hospital setting. Therefore, a comprehensive understanding of both clinical and administrative aspects is crucial for optimizing patient care and healthcare resource utilization.
Conclusions
Our study provides evidence that robotic-assisted resection of mediastinal masses, spanning all compartments, is both safe and feasible. Notably, this approach remains effective even for large masses measuring up to 21.5 cm. Our findings underscore the efficacy of RATS, characterized by minimal conversion rates, high rates of complete resection, and low postoperative complication rates, resulting in excellent clinical outcomes.
RATS is combining the advantages of VATS while overcoming its limitations. Therefore, with the rising availability of robotic platforms, great training capabilities and advantages like the three-dimensional view and articulating instruments with their excellent maneuverability, it is anticipated, that RATS will assume a pivotal role in resection of mediastinal masses. Nevertheless, the open approach still has its role, especially for complex or locally advanced tumors with a broad invasion into adjacent structures and an involvement of bigger vessels (e.g., the brachiocephalic vein or superior vena cava), where extended resection, potential reconstruction or a better control of blood flow is required.
Acknowledgments
Funding: None.
Footnote
, until 2022.
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Visualized Surgery for the series “Robotic Thoracic Surgery: Established Procedures & Current Trends”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-48/rc
Data Sharing Statement: Available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-48/dss
Peer Review File: Available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-48/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-48/coif). The series “Robotic Thoracic Surgery: Established Procedures & Current Trends” was commissioned by the editorial office without any funding or sponsorship. G.J.K. served as the unpaid Guest Editor of the series and serves as an unpaid Associate Editor-in-Chief of Journal of Visualized Surgery from March 2024 to February 2026. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical approval was not required as the research data contained no personal and sensitive data, including anonymized health-related personal data, which do not fall within the scope of the Federal Act on Research involving Human Beings (Swiss Federal Human Research Act, HRA). Written informed consent was obtained from all patients, and the study was approved by Thoracic Surgery Review Board Inselspital (approval number, TS03-2021/2; date of approval, 10 May 2021).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Priola AM, Priola SM, Cardinale L, et al. The anterior mediastinum: diseases. Radiol Med 2006;111:312-42. [Crossref] [PubMed]
- Davis RD Jr, Oldham HN Jr, Sabiston DC Jr. Primary cysts and neoplasms of the mediastinum: recent changes in clinical presentation, methods of diagnosis, management, and results. Ann Thorac Surg 1987;44:229-37. [Crossref] [PubMed]
- Duwe BV, Sterman DH, Musani AI. Tumors of the mediastinum. Chest 2005;128:2893-909. [Crossref] [PubMed]
- Strollo DC, Rosado de Christenson ML, Jett JR. Primary mediastinal tumors. Part 1: tumors of the anterior mediastinum. Chest 1997;112:511-22. [Crossref] [PubMed]
- Carter BW, Marom EM, Detterbeck FC. Approaching the patient with an anterior mediastinal mass: a guide for clinicians. J Thorac Oncol 2014;9:S102-9. [Crossref] [PubMed]
- Liu W, Deslauriers J. Mediastinal divisions and compartments. Thorac Surg Clin 2011;21:183-90. viii. [Crossref] [PubMed]
- Davenport E, Malthaner RA. The role of surgery in the management of thymoma: a systematic review. Ann Thorac Surg 2008;86:673-84. [Crossref] [PubMed]
- Hazzard C, Flores R, Nicastri DG. Mediastinal surgery: modern treatment of primary germ cell tumor of the mediastinum. Journal of Visualized Surgery; Vol 4, No 6 (June 2018) Journal of Visualized Surgery 2018; [Crossref]
- O'Sullivan KE, Kreaden US, Hebert AE, et al. A systematic review of robotic versus open and video assisted thoracoscopic surgery (VATS) approaches for thymectomy. Ann Cardiothorac Surg 2019;8:174-93. [Crossref] [PubMed]
- Augustin F, Schmid T, Bodner J. The robotic approach for mediastinal lesions. Int J Med Robot 2006;2:262-70. [Crossref] [PubMed]
- Straughan DM, Fontaine JP, Toloza EM. Robotic-Assisted Videothoracoscopic Mediastinal Surgery. Cancer Control 2015;22:326-30. [Crossref] [PubMed]
- Zirafa CC, Ricciardi S, Cavaliere I, et al. The application of robotic surgery on the anterior mediastinal tumors. Journal of Visualized Surgery; Vol 4 (September 2018) Journal of Visualized Surgery 2018; [Crossref]
- Yoshino I, Hashizume M, Shimada M, et al. Thoracoscopic thymomectomy with the da Vinci computer-enhanced surgical system. J Thorac Cardiovasc Surg 2001;122:783-5. [Crossref] [PubMed]
- Rueckert J, Swierzy M, Badakhshi H, et al. Robotic-assisted thymectomy: surgical procedure and results. Thorac Cardiovasc Surg 2015;63:194-200. [Crossref] [PubMed]
- Bodner J, Wykypiel H, Greiner A, et al. Early experience with robot-assisted surgery for mediastinal masses. Ann Thorac Surg 2004;78:259-65; discussion 265-6. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Minnich DJ. Operative techniques in robotic thoracic surgery for inferior or posterior mediastinal pathology. J Thorac Cardiovasc Surg 2012;143:1138-43. [Crossref] [PubMed]
- Li XK, Cong ZZ, Xu Y, et al. Clinical efficacy of robot-assisted thoracoscopic surgery for posterior mediastinal neurogenic tumors. J Thorac Dis 2020;12:3065-72. [Crossref] [PubMed]
- Kajiwara N, Kakihana M, Usuda J, et al. Extended indications for robotic surgery for posterior mediastinal tumors. Asian Cardiovasc Thorac Ann 2012;20:308-13. [Crossref] [PubMed]
- Chen K, Zhang X, Jin R, et al. Robot-assisted thoracoscopic surgery for mediastinal masses: a single-institution experience. J Thorac Dis 2020;12:105-13. [Crossref] [PubMed]
- Li H, Li J, Huang J, et al. Robotic-assisted mediastinal surgery: the first Chinese series of 167 consecutive cases. J Thorac Dis 2018;10:2876-80. [Crossref] [PubMed]
- Des Jarlais DC, Lyles C, Crepaz N, et al. Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: the TREND statement. Am J Public Health 2004;94:361-6. [Crossref] [PubMed]
- Wei B, Cerfolio R. Robotic thymectomy. J Vis Surg 2016;2:136. [Crossref] [PubMed]
- Detterbeck FC, Nicholson AG, Kondo K, et al. The Masaoka-Koga stage classification for thymic malignancies: clarification and definition of terms. J Thorac Oncol 2011;6:S1710-6. [Crossref] [PubMed]
- World Health Organization. WHO Classification of Tumours Editorial Board. Thoracic tumours. Lyon (France): International Agency for Research on Cancer; 2021.
- Ahmad U. The eighth edition TNM stage classification for thymic tumors: What do I need to know? J Thorac Cardiovasc Surg 2021;161:1524-9.
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Kneuertz PJ, Kamel MK, Stiles BM, et al. Robotic Thymectomy Is Feasible for Large Thymomas: A Propensity-Matched Comparison. Ann Thorac Surg 2017;104:1673-8. [Crossref] [PubMed]
- Wilshire CL, Vallières E, Shultz D, et al. Robotic Resection of 3 cm and Larger Thymomas Is Associated With Low Perioperative Morbidity and Mortality. Innovations (Phila) 2016;11:321-6. [Crossref] [PubMed]
- Azenha LF, Deckarm R, Minervini F, et al. Robotic vs. Transsternal Thymectomy: A Single Center Experience over 10 Years. J Clin Med 2021;10:4991. [Crossref] [PubMed]
- Wang M, Kundu U, Gong Y. Pitfalls of FNA diagnosis of thymic tumors. Cancer Cytopathol 2020;128:57-67. [Crossref] [PubMed]
- Girard N, Ruffini E, Marx A, et al. Thymic epithelial tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015;26:v40-55. [Crossref] [PubMed]
- Kondo K, Yoshizawa K, Tsuyuguchi M, et al. WHO histologic classification is a prognostic indicator in thymoma. Ann Thorac Surg 2004;77:1183-8. [Crossref] [PubMed]
- Yuan ZY, Gao SG, Mu JW, et al. Long-term outcomes of 307 patients after complete thymoma resection. Chin J Cancer 2017;36:46. [Crossref] [PubMed]
- Patel DC, Shrager JB, Padda SK. The role of induction therapy for thymic malignancies: a narrative review. Mediastinum 2020;4:36. [Crossref] [PubMed]
- Park S, Park IK, Kim YT, et al. Comparison of Neoadjuvant Chemotherapy Followed by Surgery to Upfront Surgery for Thymic Malignancy. Ann Thorac Surg 2019;107:355-62. [Crossref] [PubMed]
- Lucchi M, Melfi F, Dini P, et al. Neoadjuvant chemotherapy for stage III and IVA thymomas: a single-institution experience with a long follow-up. J Thorac Oncol 2006;1:308-13. [Crossref] [PubMed]
- No TH, Seol SH, Seo GW, et al. Benign Mature Teratoma in Anterior Mediastinum. J Clin Med Res 2015;7:726-8. [Crossref] [PubMed]
- Wang R, Li H, Jiang J, et al. Incidence, treatment, and survival analysis in mediastinal malignant teratoma population. Transl Cancer Res 2020;9:2492-502. [Crossref] [PubMed]
- Bokemeyer C, Droz JP, Horwich A, et al. Extragonadal seminoma: an international multicenter analysis of prognostic factors and long term treatment outcome. Cancer 2001;91:1394-401. [Crossref] [PubMed]
- Napieralska A, Majewski W, Osewski W, et al. Primary mediastinal seminoma. J Thorac Dis 2018;10:4335-41. [Crossref] [PubMed]
- Marandino L, Vogl UM. Mediastinal germ cell tumours: where we are and where we are going-a narrative review. Mediastinum 2022;6:7. [Crossref] [PubMed]
- Bouma W, Klinkenberg TJ, Van De Wauwer C, et al. Removal of a giant intrathoracic cyst from the anterior mediastinum. J Cardiothorac Surg 2014;9:152. [Crossref] [PubMed]
- McAdams HP, Kirejczyk WM, Rosado-de-Christenson ML, et al. Bronchogenic cyst: imaging features with clinical and histopathologic correlation. Radiology 2000;217:441-6. [Crossref] [PubMed]
- Crapo JD, Glassroth J, Karlinsky JB, et al. Baum's Textbook of Pulmonary Diseases. Philadelphia: Lippincott Williams & Wilkins; 2003.
- Strollo DC, Rosado-de-Christenson ML, Jett JR. Primary mediastinal tumors: part II. Tumors of the middle and posterior mediastinum. Chest 1997;112:1344-57. [Crossref] [PubMed]
- Chen X, Ma Q, Wang S, et al. Surgical treatment of posterior mediastinal neurogenic tumors. J Surg Oncol 2019;119:807-13. [Crossref] [PubMed]
- Shrivastava V, Vundavalli S, Smith D, et al. A chondroma of the anterior mediastinum. Clin Radiol 2006;61:1065-6. [Crossref] [PubMed]
- Vuckovic DC, Koledin MP, Vuckovic NM, et al. Mediastinal Cartilaginous Hamartoma. Cureus 2020;12:e7411. [PubMed]
- Chick JF, Chauhan NR, Madan R. Solitary fibrous tumors of the thorax: nomenclature, epidemiology, radiologic and pathologic findings, differential diagnoses, and management. AJR Am J Roentgenol 2013;200:W238-48. [Crossref] [PubMed]
- Dispenzieri A, Fajgenbaum DC. Overview of Castleman disease. Blood 2020;135:1353-64. [Crossref] [PubMed]
- Li R, Ma Z, Qu C, et al. Comparison of perioperative outcomes between robotic-assisted and video-assisted thoracoscopic surgery for mediastinal masses in patients with different body mass index ranges: A population-based study. Front Surg 2022;9:963335. [Crossref] [PubMed]
- Agatsuma H, Yoshida K, Yoshino I, et al. Video-Assisted Thoracic Surgery Thymectomy Versus Sternotomy Thymectomy in Patients With Thymoma. Ann Thorac Surg 2017;104:1047-53. [Crossref] [PubMed]
- Burt BM, Yao X, Shrager J, et al. Determinants of Complete Resection of Thymoma by Minimally Invasive and Open Thymectomy: Analysis of an International Registry. J Thorac Oncol 2017;12:129-36. [Crossref] [PubMed]
- Fok M, Bashir M, Harky A, et al. Video-Assisted Thoracoscopic Versus Robotic-Assisted Thoracoscopic Thymectomy: Systematic Review and Meta-analysis. Innovations (Phila) 2017;12:259-64. [Crossref] [PubMed]
- Alvarado CE, Worrell SG, Bachman KC, et al. Robotic Approach Has Improved Outcomes for Minimally Invasive Resection of Mediastinal Tumors. Ann Thorac Surg 2022;113:1853-8. [Crossref] [PubMed]
- Shen C, Li J, Li J, et al. Robot-assisted thoracic surgery versus video-assisted thoracic surgery for treatment of patients with thymoma: A systematic review and meta-analysis. Thorac Cancer 2022;13:151-61. [Crossref] [PubMed]
Cite this article as: Grawunder D, Flury DV, Deckarm S, Kocher GJ. Robotic resection of mediastinal masses: a decade of experience. J Vis Surg 2024;10:7.