Uniportal & biportal robotic anatomic lung resection (without CO2 insufflation): technique, initial experience and cost
Highlight box
Key findings
• Performing anatomic lung resections through uniportal robotic-assisted thoracic surgery (uRATS) and biportal RATS (bRATS) without carbon dioxide insufflation is a safe and feasible approach that yields good perioperative results.
• Both approaches are viable and demonstrate comparable perioperative outcomes.
• Due to enhanced maneuverability, visualization and ergonomics, RATS allows a profound lymphadenectomy with overall low blood loss and low conversion rates.
• The costs for RATS are currently higher compared to video-assisted thoracic surgery.
What is known and what is new?
• Since RATS was introduced two decades ago it marks a steady increase in utilization for anatomic lung resections.
• Our data supports the good perioperative outcomes of RATS reported in literature and highlights the current advantages and disadvantages of uRATS and bRATS anatomic lung resections.
What is the implication, and what should change now?
• With the increasing popularity of robotics among thoracic surgeons worldwide, the outlook for RATS anatomic lung resection in the future is very promising, with more research and development expected to improve the technology and its applications, especially for uRATS.
Introduction
With globally 2.3 million newly diagnosed lung cancer cases and 1.8 million deaths annually, lung cancer is the most common cause of cancer-related deaths worldwide and is responsible for the highest mortality rate in men and women (1). Non-small cell lung cancer (NSCLC) is the most common type of lung cancer and accounts for 85% of all lung cancer cases. Adenocarcinomas represent 40% of all NSCLC, while 25–30% are squamous-cell carcinomas and 5–10% are large cell carcinomas (2).
Minimally invasive radical resection with lymphadenectomy is the current standard approach for all types of NSCLC in stage I as well as for stage II and a feasible approach for stage IIIA NSCLC in a combined multimodality approach, e.g., following neoadjuvant therapy (2).
Currently, the most established surgical approach is video-assisted thoracic surgery (VATS), which shows significant advantages over conventional thoracotomy in terms of perioperative complications, length of hospital stay (LOS) and mortality rate, while maintaining similar oncologic outcomes and long-term survival (3-7). VATS can be performed by either a multiport technique with three or four ports or by the uniportal approach (uVATS), in which the camera and instruments are inserted through only one single small muscle-sparing incision. Uniportal VATS has been shown to have similar surgical outcomes compared to multiport VATS, while additionally improving the patient’s short-term quality of life, especially regarding postoperative pain due to involvement of only one intercostal space (8,9).
The transition from open surgery to VATS required adaptation to a reduced two-dimensional visual field, smaller incisions limiting the range of instrument movement and an increased instrument length, which amplifies tremor. These conditions are associated with a more complex learning curve (10,11).
Robotic-assisted thoracic surgery (RATS) partially overcomes the limitations of VATS by providing the surgeon with a three-dimensional visual field, a 360-degree range of instrument movement and tremor suppression.
Since its first use over three decades ago, robotic-assisted surgery became the standard approach for certain surgical procedures and is regularly used, among others, in the fields of urology, gynecology, otorhinolaryngology, cardiac and general surgery (12).
While first applied in thoracic surgery two decades ago, different studies over the past 10 years confirmed the safety of RATS, which led to a steady increase in its utilization for anatomic lung resections (13-16). Similar to VATS, the initial approach was multiportal (mRATS) with four incisions (one camera port and three utility ports). The introduction of the da Vinci Xi system (Intuitive Surgical, Sunnyvale, CA, USA) allowed to reduce the number of ports from four to two (biportal RATS; bRATS) and finally from two to one (uniportal RATS; uRATS) (17). Due to slender arms and 8 mm trocars, the uniportal approach could be established. Additionally, in 2018 the da Vinci SP (Single Port) (Intuitive Surgical) system was introduced, which is especially designed for uniportal usage, as the camera and three instruments are inserted through a single trocar and can be utilized independently intrathoracically. First attempts have been made to utilize the da Vinci SP platform in thoracic surgery and it is routinely used in urology and otorhinolaryngology (17,18). However, the single-port da Vinci SP system exhibits several distinctions when compared to its multi-port counterpart, the da Vinci Xi system. A primary divergence is the absence of an integrated robotic stapler in the da Vinci SP system. Consequently, thoracoscopic stapling necessitates manual operation by the surgical assistant, who must work in conjunction with the single-port system via the identical single access port. Furthermore, the physical dimensions of the da Vinci SP system preclude its deployment through the intercostal space. This restriction confines its use to subxiphoidal (subcostal) approaches exclusively, a limitation not shared by the more versatile da Vinci Xi system (19). This highlights the distinct features and limitations of the single-port da Vinci SP system in robotic-assisted surgery. However, it is important to note that ongoing advancements could potentially expand its application in uRATS in the future.
Initial experiences reported in the literature indicate that the utilization of the da Vinci Xi system for anatomical lung resection in RATS yields promising results, even in the context of advanced procedures such as sleeve resections (20-24).
The learning curve of RATS has been reported to be similar than VATS, although surgeons with previous VATS experience might benefit from a more intuitive experience and rapid increase in expertise (25,26).
mRATS and multiportal VATS, as regularly used in visceral surgery, and some thoracic procedures, e.g., thymectomy, sympathectomy and anatomic lung resections, are typically performed with insufflation of carbon dioxide (CO2), requiring each trocar to have an air seal (27).
Uniportal VATS and uRATS can be performed without the use of CO2 insufflation, as selective one-lung ventilation is usually sufficient for surgical exposure. Although silicon pads are available for the uniportal approach and allow the usage of CO2 insufflation, it is reported that the movement of instruments is less flexible using silicon pads for air sealing compared with the standard uniportal approach. Additionally, the use of CO2 insufflation can be associated with the risk of complications such as CO2 embolism, hemodynamic alterations such as decreased venous return and bradycardia and acid-base disorders with arterial hypoxia (28). However, it is crucial to underscore that these risks are substantially mitigated when overpressure conditions are meticulously avoided, thereby significantly reducing the probability of associated complications. We present this article in accordance with the TREND reporting checklist (available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-47/rc).
Methods
In this article we present data of the first 13 consecutive patients who underwent uRATS or bRATS anatomic lung resection without the use of CO2 insufflation at the Department of Thoracic Surgery St. Claraspital Basel and Hirslanden Clinic Beau-Site & Lindenhof Bern in 2023.
All patients underwent standard preoperative examination as well as staging according to the current guidelines.
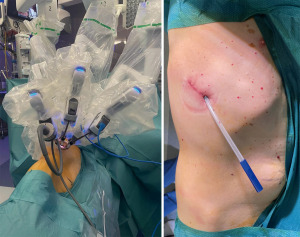
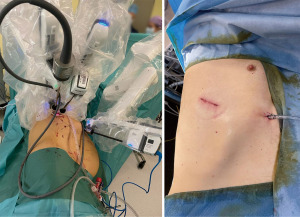
The standard uniportal robotic set-up requires one incision of 3–5 cm with three robotic arms inserted through 8–12 mm trocars. One trocar is utilized for the camera, as the other two arms are used as working arms for the energy device or stapler as well as a grasper (17). The set-up for uRATS is shown in Figure 1 and the intraoperative set-up for a biportal robotic-assisted approach is shown in Figure 2.
Statistical analysis
Categorical variables were compared using two-tailed t-test for independent samples, Chi-Square test and Fischer’s exact test. Data is reported as mean and standard deviations. Statistical calculations were made using SPSS Version 29.0.0 (www.ibm.com, IBM®).
Ethics consideration
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical approval was not required as the research data contained no personal and sensitive data, including anonymized health-related personal data, which do not fall within the scope of the Federal Act on Research involving Human Beings (Swiss Federal Human Research Act, HRA). Informed consent was obtained from all patients.
Results
Data of the first 13 patients (n=13) undergoing robotic-assisted anatomic lung resection at the Department of Thoracic Surgery St. Claraspital Basel and Hirslanden Clinic Beau-Site & Lindenhof Bern in 2023 was analyzed. Patient characteristics including age and gender as well as the approach are shown in Table 1. Five (38.46%) patients underwent pure uRATS and in eight (61.54%) an additional 12 mm port was used (bRATS) for the use of the robotic stapler (subxiphoid position in right sided resections and as anterior as possible in left sided resections).
Table 1
| Characteristics | Total | RATS approach | |
|---|---|---|---|
| Uniportal | Biportal | ||
| Age (years) | 71.62±9.62 | 63±10.91 | 77±4.21 |
| Sex | |||
| M | 9 (69.23) | 4 (80.00) | 5 (62.50) |
| F | 4 (30.77) | 1 (20.00) | 3 (37.50) |
RATS, robotic-assisted thoracic surgery; M, male; F, female.
Figure 3 demonstrates the overall distribution of anatomic lung resections performed as well as the resected segments or lobes. Table 2 shows all segmentectomies and lobectomies performed for each approach. Of all performed resections, segmentectomy was performed in 10 (76.92%) of the cases and in 3 (23.08%) cases lobectomy was performed. Most performed resections were apical segmentectomies including right segment 1 (n=2), right segment 2 (n=2) and apical trisegmentectomy (n=2).
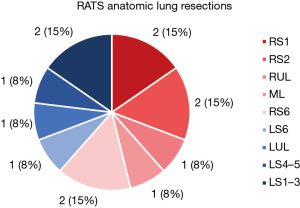
Table 2
| Resections | Total | RATS approach | |
|---|---|---|---|
| Uniportal | Biportal | ||
| Anatomic lung resections | 13 | 5 (38.46) | 8 (61.54) |
| Lobectomies | 3 (23.08) | 1 (20.00) | 2 (25.00) |
| Right upper lobe | 1 | – | 1 |
| Middle lobe | 1 | – | 1 |
| Left upper lobe | 1 | 1 | – |
| Segmentectomies | 10 (76.92) | 4 (80.00) | 6 (75.00) |
| Right S1 | 2 | – | 2 |
| Right S2 | 2 | 1 | 1 |
| Right S6 | 2 | 1 | 1 |
| Left S1–3 | 2 | 1 | 1 |
| Left S4–5 | 1 | 1 | – |
| Left S6 | 1 | – | 1 |
RATS, robotic-assisted thoracic surgery; S1, segment 1; S2, segment 2; S6, segment 6; S1–3, segment 1–3 (apical trisegments); S4–5, segment 4–5 (lingula).
Figure 4 shows, that 81.82% (n=9) of all resections performed in patients with lung cancer were postoperatively staged as stage I, 9.09% (n=1) had postoperative stage IIB and 9.09% (n=1) showed postoperative stage IIIA due to mediastinal lymph node involvement.
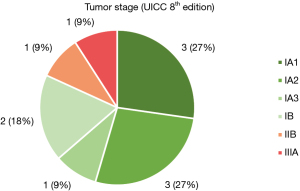
Table 3 shows the total number of anatomic lung resections, segmentectomies as well as lobectomies performed associated with the postoperative lung cancer stage. Table 4 shows the postoperative histopathological results. In one case, postoperative histopathologic evaluation showed intrapulmonary infiltration of amyloidosis and in another case infiltration of sarcoidosis. In 76.92% of patients (n=10), there was histopathological confirmation of NSCLC, of which 90% (n=9) were adenocarcinomas and 10% (n=1) squamous cell carcinomas. In one case (7.69%) histopathologic results confirmed a neuroendocrine tumor (typical carcinoid) and in two cases (15.38%) no malignancy was found.
Table 3
| p-TNM stage (lung cancer) | Total (n=13) | Type of anatomic lung resection | |
|---|---|---|---|
| Segmentectomy (n=10) | Lobectomy (n=3) | ||
| IA1 | 3 | 3 | 0 |
| IA2 | 3 | 3 | 0 |
| IA3 | 1 | 0 | 1 |
| IB | 2 | 2 | 0 |
| IIB | 1 | 0 | 1 |
| IIIA | 1 | 0 | 1 |
| – | 2 | 2 | 0 |
N=2 showed no malignancy. UICC, Union for International Cancer Control; p-TNM, pathological tumor/node/metastasis staging.
Table 4
| Histopathologic findings | Total |
|---|---|
| NSCLC | 10 (76.92) |
| Adenocarcinoma | 9 (90.00) |
| Squamous cell carcinoma | 1 (10.00) |
| Neuroendocrine tumor | 1 (7.69) |
| Typical carcinoid | 1 (100.00) |
| Benign | 2 (15.38) |
| Amyloidosis | 1 (50.00) |
| Sarcoidosis | 1 (50.00) |
NSCLC, non-small cell lung cancer.
The mean operating time was 136.38±39.56 min for all RATS anatomic lung resections without significant difference between the uRATS (mean: 142.40±49.50 min) and the bRATS group (mean: 132.63±38.27 min) (P=0.69), as shown in Table 5. No intraoperative complications were encountered. Thus, we report zero conversions to VATS or thoracotomy. Mean blood loss was low with 27.85±30.48 mL, without significant difference between the uRATS (mean: 46.40±45.11 mL) and the bRATS group (mean: 16.25±12.75 mL) (P=0.09). Numbers of resected lymph nodes with a mean of 16.00±12.34 lymph nodes were similar in both groups without significant difference (uRATS mean: 11.00±5.57, bRATS mean: 18.14±15.02) (P=0.45).
Table 5
| Outcomes | Total | RATS approach | P value | |
|---|---|---|---|---|
| Uniportal | Biportal | |||
| Duration (min) | 136.38±39.56 | 142.40±49.50 | 132.63±38.27 | 0.69 |
| Blood loss (mL) | 27.85±30.48 | 46.40±45.11 | 16.25±12.75 | 0.09 |
| Number of resected LNs | 16.00±12.34 | 11.00±5.57 | 18.14±15.02 | 0.45 |
| Chest tube removal (POD) (days) | 3.69±2.78 | 3.40±1.52 | 3.88±3.60 | 0.78 |
| LOS (days) | 4.62±1.90 | 4.00±1.22 | 5.00±2.33 | 0.33 |
| Conversion | 0 | 0 | 0 | – |
RATS, robotic-assisted thoracic surgery; LNs, lymph nodes; POD, postoperative day; LOS, length of hospital stay.
Mean postoperative chest tube removal was after 3.69±2.78 days (uRATS mean: 3.40±1.52 days, bRATS mean: 3.88±3.60 days) (P=0.78). Mean LOS was 4.62±1.90 days (uRATS mean: 4.00±1.22 days, bRATS mean: 5.00±2.33 days) (P=0.33). All patients could be discharged home, without any patient requiring postoperative rehabilitation in an inpatient setting.
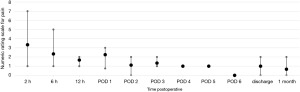
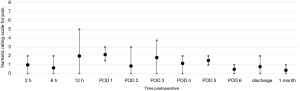
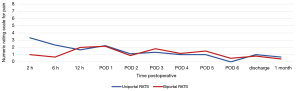
Postoperative pain was assessed through the numerical rating scale (NRS). At the end of each operation, an intercostal block was performed with ropivacaine (5 mg/mL). The postoperative NRS reported by the patients were very similar for uRATS (Figure 5) and bRATS (Figure 6), with a slight difference noted in the average pain reported 2 hours postoperative (Figure 7). As postoperative days (PODs) progressed, both procedures showed a decreasing trend in pain, with minimal pain reported at discharge and at the clinical follow-up 1 month postoperative (Figure 7).
All intraoperative and postoperative complications during the first 30 postoperative days were recorded and evaluated using the Clavien-Dindo grading system and are shown in Table 6. During the postoperative stay, one patient developed a prolonged air leak, requiring discharge with a chest tube on POD 7 and removal thereof on POD 12 in an outpatient setting. One patient in the bRATS group showed multiple postoperative complications [atrial fibrillation and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-infection], both required medications. No patient required re-operation or surgical intervention. There was no statistically significant relationship between uniportal or biportal approach and the presence of complications (yes/no) (P=0.83) as well as the specific postoperative complications (P=0.41).
Table 6
| Complications | Total | RATS approach | |
|---|---|---|---|
| Uniportal | Biportal | ||
| Anatomic lung resections | 13 | 5 | 8 |
| Patients with complications | 3 (23.08) | 1 (20.00) | 2 (25.00) |
| Postoperative complications | |||
| Clavien Dindo Grade I | 1 | – | 1 |
| Prolonged air leak (>5 d) | 1 | – | 1 |
| Clavien Dindo Grade II | 3 | 1 | 2 |
| Atrial fibrillation | 2 | 1 | 1 |
| SARS-CoV-2-infection | 1 | – | 1 |
RATS, robotic-assisted thoracic surgery; d, days; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Mean earnings before interest, taxes, depreciation, and amortization (EBITDA) for robotic-assisted resections was 1,809.5 CHF (Swiss francs), while mean EBITDA for uniportal VATS was 2,907.1 CHF over the last 12 months, meaning RATS is costlier with a current difference in EBITDA of 1,097.6 CHF.
Discussion
Compared to VATS lobectomy and segmentectomy, RATS has been repeatedly shown to be non-inferior or better in terms of duration of operation, perioperative blood loss, conversion rate, postoperative chest tube duration, LOS, overall postoperative complications, mortality, re-intervention, overall survival, and disease-free survival (30-32). Large retrospective studies have confirmed low rates of major complications after RATS anatomic lung resections, with the most common complications including prolonged air leak, pneumonia, atelectasis and atrial arrhythmia (33,34). In our cohort, both uRATS and bRATS showed comparable rates of complications, with complications (only Clavien-Dindo stage I to II) (35) occurring in three out of 13 patients (23.08%).
The duration of hospitalization is highly dependent on chest tube duration, overall chest tube management, postoperative pain and presence of complications. Literature shows similar data regarding LOS and chest tube duration in uRATS compared to uVATS, but it is important to note that most specialized centers have their own postoperative criteria for chest tube removal, which might impair data comparability between centers (36).
Additionally, due to an increased implementation and standardization of screening procedures, earlier stages of NSCLC are expected to be detected. In Switzerland, and generally in Europe, a wide-spread screening implementation is expected to be implemented in the coming decades. Currently, in Europe no uniform recommended screening protocol has been established, but data from the United States (US) showed evidence of higher lung cancer incidence at 6.5-year follow-up in patients who received low-dose computed tomography compared to chest X-rays, as well as a stage shift with more stage I and stage II and less stage III and stage IV diagnoses of lung cancer (37). With early detection of lung cancer, which often results in the identification of smaller tumors, more lung sparing resections can be performed, which also leads to reduced surgical trauma (38,39). For NSCLC in early stages with a limited tumor size of ≤2 cm, anatomic lung resections in the form of sublobar segmentectomies were shown to be non-inferior to lobectomy regarding disease-free survival and show similar overall survival (40,41). This necessitates a transition towards more precise minimally invasive lung surgery (sublobar resections), thereby amplifying the relevance of RATS in the coming decades. This is a trend that has already been observed in certain reports, with RATS sublobar resections reported to be safe and feasible (42-44).
RATS may unfold its full potential especially for more complex procedures, such as sleeve resections or en-bloc chest wall resection (e.g., in Pancoast tumors) (45,46). RATS may also be beneficial due to its advantages for anatomic segmentectomies as these present increased complexity due to intraparenchymal dissection, reduction of vessel and bronchial size, more frequent anatomical variants and the challenge of defining the intersegmental plane for sublobar parenchymal resection (38).
RATS with its three-dimensional magnified view and refined maneuverability allows for more surgical precision compared to traditional VATS. In our cohort, segmentectomies were preferred whenever the tumor size was ≤2 cm. Hence, segmentectomies represent the majority of resections performed. For larger tumors and depending on tumor stage, lobectomy was performed, according to the current guidelines.
A shift from multiport RATS to uRATS or bRATS has been observed in recent studies and shows comparable perioperative and postoperative results including conversion rates, blood loss, hospital stay and postoperative complications (47). Additionally, both show low postoperative pain and good cosmetic results.
Pure uRATS is RATS performed through a single intercostal incision, without rib spreading, using the robotic camera, robotic dissecting instruments and robotic staplers, all controlled through the surgeon console.
uRATS can be performed with the use of conventional handheld staplers and a uniportal access usually in the sixth intercostal space, or as pure uRATS with the use of robotic staplers with an access usually in the seventh intercostal space. Although the robotic stapler offers a better maneuverability compared to the handheld stapling device, the joint of the robotic stapler has to leave the trocar to be able to move. Hence, this might require more distance between the incision (intercostal space) and the structure that needs to be controlled and divided. Therefore, an additional port, offering a wider intercostal space (to harbor the 12 mm stapler trocar) and a lower intercostal space (or even subxiphoid position for right sided resections) might be more suitable (17). Like this, crowding of instruments in the intercostal space can also be avoided and tension as well as trauma in the intercostal space due to overlapping of the trocars can be reduced. The assistant port in bRATS is typically used for one of the robotic arms, especially for stapling. This leaves more room in the intercostal space of the main incision to pass a suction or grasper in between the two robotic arms (camera and instrument).
The most common reasons for choosing a biportal approach in our cohort included limited intercostal width and/or the requirement to create a better distance from the port to the area of dissection when intending to use the robotic stapler.
Due to currently limited utilization of the uniportal and biportal robotic technique for anatomic lung resections, data on operating time and complications is still limited. Conflicting results have been reported on operating time using uRATS vs. mRATS (30,36,47).
Compared to bRATS, uRATS is reported to show lower postoperative pain (Visual Analogue Scale; VAS) scores (30). Our results indicate similar postoperative pain for both approaches. Nonetheless, the interpretation of these findings necessitates the consideration of several critical factors. The inherently subjective nature of pain perception introduces variability into the average pain scores reported by individual patients. Additionally, the presence of pre-existing chronic pain in some patients and the general nature of pain assessment conducted by nursing staff, which often encompasses overall rather than surgical-area-specific pain, may confound the results. Furthermore, the use of pre-existing pain medication by some patients due to chronic pain can complicate postoperative pain management. In addition, patient-specific factors such as intolerance to certain analgesics or the presence of comorbidities may limit the range of available analgesics, introducing another layer of complexity. These considerations underscore the need for a nuanced approach to interpreting the comparative efficacy of uRATS and bRATS in managing postoperative pain. In summary, a methodologically rigorous approach that incorporates objective measures, accounts for contextual factors, and employs standardized tools is crucial for meaningful comparisons of postoperative pain outcomes between different surgical approaches. By implementing these strategies, we can enhance the reliability and validity of pain score comparisons in surgical research.
The rate of prolonged postoperative air leak is reported to be significantly lower when a uniportal approach is employed, as opposed to a multiportal approach. However, these findings may be influenced by confounding variables such as the presence of adhesions and other factors that contribute to the complexity of the surgical procedure (47). The abovementioned results are consistent with our findings. Both robotic approaches theoretically allow a fast conversion to VATS or thoracotomy although in case of major intraoperative bleeding, uRATS might be superior to mRATS, as the trocars can be disconnected more rapidly and allow for faster conversion to uVATS or thoracotomy and achievement of hemostasis (47). The outcomes of the surgical procedure are highly influenced, among others, by the extent of the disease, patient anatomy and variants, and the surgeon’s experience. Additionally, some authors also recommend the biportal method as a useful transition stage from the initial multiportal to uniportal robotic-assisted anatomic lung resections (47).
While the patients included in our study undoubtedly represent a carefully selected group of patients with early tumor stages, resectable tumors and given operability, demographically we can see a clear overlap with reported NSCLC populations. Currently mean age at diagnosis is reported to be around 70 years by several national registries in Europe and the US, which is consistent with the mean age of our patient cohort (48,49). Additionally, the incidence of lung cancer was historically higher in males than females, as it is the case in our cohort. Yet, in the past decades incidence rates converged and crossed over in specific age groups in several countries. This is partially attributable to changes of modifiable risk factors such as smoking habits but is not yet fully explained.
NSCLC may be categorized into different subgroups, with the most frequent being adenocarcinomas (40%) and squamous cell carcinomas (25%) (50-52). While adenocarcinomas are overrepresented our cohort, the typical location of NSCLC in the upper pulmonary lobes is consistent with reported data (53,54). Carcinogenic factors responsible for this distribution have not been definitively proven, though genetic and local factors are thought to contribute to this distribution pattern.
The major factor making RATS seem feasible from an oncological standpoint is the extent and completion of lymphadenectomy due to increased range of motion and maneuverability of the surgical instruments. While results are not yet conclusive, using a robotic approach may result in higher quantity of resected lymph nodes compared to a video-assisted approach. Nevertheless, no difference in upstaging the N-factor could be identified (15,32,55-60). European Society for Medical Oncology (ESMO) guidelines for early and locally advanced NSCLC recommend mediastinal lymph node sampling (MLNS) from six stations with three hilar and three mediastinal stations including the subcarinal space (61). Whether complete mediastinal lymph node dissection (MLND) or MLNS is the preferred approach is a topic of debate (62-64).
Comparing costs between VATS and RATS, especially for uniportal or biportal approaches, is difficult. Most conducted studies use varying definitions, methodologies and cost basis (e.g., depreciation costs, postoperative costs) (65). Comparability between studies is further hindered by the different conducted operations including lobectomies and segmentectomies or more complex procedures (66). In our study we report the EBITDA, which requires consideration of several factors, including the procedure, diagnosis, insurance status, postoperative stay on the intensive care unit (ICU) and in-hospital procedures including blood work, among others. Our cost calculation supports the findings that RATS is costlier than VATS and is thus consistent with most reported literature on this topic (56,67-69). Even in a diagnosis-related group (DRG)-driven healthcare system like Switzerland’s, hospitals can still earn money from robotic surgery for anatomic lung resections.
This study is limited as it is a retrospective study with a limited patient population. Rigorous multicenter randomized prospective studies are required to further evaluate this issue. A better understanding of patient characteristics for selecting patients that are suitable for RATS lung resection and identifying predictors may be valuable. Additionally, further follow-up for long-term outcomes is necessary.
Conclusions
uRATS and bRATS are feasible approaches for anatomic lung resection. Our data shows no intraoperative and low postoperative complication rates. In our experience, occasionally a second port (bRATS) was necessary and useful for originally intended uRATS approaches, however, the outcomes were not significantly affected by the different approaches (uRATS vs. bRATS). We do not report significant differences in perioperative complications and LOS between the uRATS or bRATS approach. Between the two groups operating time, blood loss, numbers of resected lymph nodes, chest tube removal and length of stay were similar. The cost for RATS at our institution was higher compared to conventional uniportal VATS.
Biportal approaches may be a valuable transitional step in the process of adopting uRATS for anatomic lung resection in resectable NSCLC, especially when robotic stapling is used.
Further research is needed to determine the optimal approach for robotic-assisted anatomic lung resections and more rigorous trials comparing uniportal and biportal approaches may provide valuable insights for the future.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Visualized Surgery for the series “Robotic Thoracic Surgery: Established Procedures & Current Trends”. The article has undergone external peer review.
Reporting Checklist: The authors have completed the TREND reporting checklist. Available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-47/rc
Data Sharing Statement: Available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-47/dss
Peer Review File: Available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-47/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-47/coif). The series “Robotic Thoracic Surgery: Established Procedures & Current Trends” was commissioned by the editorial office without any funding or sponsorship. G.J.K. served as the unpaid Guest Editor of the series and serves as an unpaid Associate Editor-in-Chief of Journal of Visualized Surgery from March 2024 to February 2026. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Ethical approval was not required as the research data contained no personal and sensitive data, including anonymized health-related personal data, which do not fall within the scope of the Federal Act on Research involving Human Beings (Swiss Federal Human Research Act, HRA). Informed consent was obtained from all patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Zappa C, Mousa SA. Non-small cell lung cancer: current treatment and future advances. Transl Lung Cancer Res 2016;5:288-300. [Crossref] [PubMed]
- Falcoz PE, Puyraveau M, Thomas PA, et al. Video-assisted thoracoscopic surgery versus open lobectomy for primary non-small-cell lung cancer: a propensity-matched analysis of outcome from the European Society of Thoracic Surgeon database. Eur J Cardiothorac Surg 2016;49:602-9. [Crossref] [PubMed]
- Ghaly G, Kamel M, Nasar A, et al. Video-Assisted Thoracoscopic Surgery Is a Safe and Effective Alternative to Thoracotomy for Anatomical Segmentectomy in Patients With Clinical Stage I Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;101:465-72; discussion 472. [Crossref] [PubMed]
- Cao C, Zhu ZH, Yan TD, et al. Video-assisted thoracic surgery versus open thoracotomy for non-small-cell lung cancer: a propensity score analysis based on a multi-institutional registry. Eur J Cardiothorac Surg 2013;44:849-54. [Crossref] [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62. [Crossref] [PubMed]
- Xu W, Xu C, Ding C, et al. Comparison of the Clinical Effect of Uniportal Video-assisted Thoracoscopic Lobectomy and Biportal Video-assisted Thoracoscopic Lobectomy in the Treatment of Lung Cancer. Zhongguo Fei Ai Za Zhi 2020;23:561-7. [PubMed]
- Tsubokawa N, Harada H, Takenaka C, et al. Comparison of Postoperative Pain after Different Thoracic Surgery Approaches as Measured by Electrical Stimulation. Thorac Cardiovasc Surg 2015;63:519-25. [Crossref] [PubMed]
- Velez-Cubian FO, Ng EP, Fontaine JP, et al. Robotic-Assisted Videothoracoscopic Surgery of the Lung. Cancer Control 2015;22:314-25. [Crossref] [PubMed]
- Arad T, Levi-Faber D, Nir RR, et al. The learning curve of video-assisted thoracoscopic surgery (VATS) for lung lobectomy—a single Israeli center experience. Harefuah 2012;151:261-5, 320. [PubMed]
- Shah J, Vyas A, Vyas D. The History of Robotics in Surgical Specialties. Am J Robot Surg 2014;1:12-20. [Crossref] [PubMed]
- Melfi FM, Menconi GF, Mariani AM, et al. Early experience with robotic technology for thoracoscopic surgery. Eur J Cardiothorac Surg 2002;21:864-8. [Crossref] [PubMed]
- Nelson DB, Mehran RJ, Mitchell KG, et al. Robotic-Assisted Lobectomy for Non-Small Cell Lung Cancer: A Comprehensive Institutional Experience. Ann Thorac Surg 2019;108:370-6. [Crossref] [PubMed]
- Zhiqiang W, Shaohua M. Perioperative outcomes of robotic-assisted versus video-assisted thoracoscopic lobectomy: A propensity score matched analysis. Thorac Cancer 2023;14:1921-31. [Crossref] [PubMed]
- Grawunder D, Flury DV, Deckarm S, et al. Robotic resection of mediastinal masses: a decade of experience. J Vis Surg 2024; in press.
- Gonzalez-Rivas D, Bosinceanu M, Manolache V, et al. Uniportal fully robotic-assisted major pulmonary resections. Ann Cardiothorac Surg 2023;12:52-61. [Crossref] [PubMed]
- Gonzalez-Rivas D, Ismail M. Subxiphoid or subcostal uniportal robotic-assisted surgery: early experimental experience. J Thorac Dis 2019;11:231-9. [Crossref] [PubMed]
- Oh DS. Innovations in robotic surgery and recent developments in the SP platform. Ann Cardiothorac Surg 2023;12:126-7. [Crossref] [PubMed]
- Gonzalez-Rivas D, Bosinceanu M, Manolache V, et al. Uniportal fully robotic-assisted sleeve resections: surgical technique and initial experience of 30 cases. Ann Cardiothorac Surg 2023;12:9-22. [Crossref] [PubMed]
- Stamenovic D, Schiller P, Karampinis I, et al. Uniportal robotic assisted surgery for anatomical lung resection-First German experience. Int J Med Robot 2023; Epub ahead of print. [Crossref] [PubMed]
- Mercadante E, Martucci N, De Luca G, et al. Early experience with uniportal robotic thoracic surgery lobectomy. Front Surg 2022;9:1005860. [Crossref] [PubMed]
- Vincenzi P, Lo Faso F, Eugeni E, et al. Uniportal robotic-assisted thoracoscopic surgery for early-stage lung cancer with the Da Vinci Xi: Initial experience of two cases. Int J Med Robot 2023;19:e2477. [Crossref] [PubMed]
- Yang Y, Song L, Huang J, et al. A uniportal right upper lobectomy by three-arm robotic-assisted thoracoscopic surgery using the da Vinci (Xi) Surgical System in the treatment of early-stage lung cancer. Transl Lung Cancer Res 2021;10:1571-5. [Crossref] [PubMed]
- Veronesi G. Robotic lobectomy and segmentectomy for lung cancer: results and operating technique. J Thorac Dis 2015;7:S122-30. [PubMed]
- Andersson SE, Ilonen IK, Pälli OH, et al. Learning curve in robotic-assisted lobectomy for non-small cell lung cancer is not steep after experience in video-assisted lobectomy; single-surgeon experience using cumulative sum analysis. Cancer Treat Res Commun 2021;27:100362. [Crossref] [PubMed]
- Gallego-Poveda J, Guerra NC, Carvalheiro C, et al. Use of CO(2) in video assisted thoracic surgery and single-lumen endotracheal tube-a new less invasive approach. J Thorac Dis 2017;9:903-6. [Crossref] [PubMed]
- Stanley MD, Sancheti MS. Management of Complications in Robotic Thoracic Surgery. Thorac Surg Clin 2023;33:19-24. [Crossref] [PubMed]
- Detterbeck FC, Boffa DJ, Kim AW, et al. The Eighth Edition Lung Cancer Stage Classification. Chest 2017;151:193-203.
- Ning Y, Chen Z, Zhang W, et al. Short-term outcomes of uniportal robotic-assisted thoracic surgery anatomic pulmonary resections: experience of Shanghai Pulmonary Hospital. Ann Cardiothorac Surg 2023;12:117-25. [Crossref] [PubMed]
- Kneuertz PJ, Abdel-Rasoul M, D’Souza DM, et al. Segmentectomy for clinical stage I non-small cell lung cancer: National benchmarks for nodal staging and outcomes by operative approach. Cancer 2022;128:1483-92. [Crossref] [PubMed]
- Ma J, Li X, Zhao S, et al. Robot-assisted thoracic surgery versus video-assisted thoracic surgery for lung lobectomy or segmentectomy in patients with non-small cell lung cancer: a meta-analysis. BMC Cancer 2021;21:498. [Crossref] [PubMed]
- Cao C, Louie BE, Melfi F, et al. Outcomes of major complications after robotic anatomic pulmonary resection. J Thorac Cardiovasc Surg 2020;159:681-6. [Crossref] [PubMed]
- Reddy RM, Gorrepati ML, Oh DS, et al. Robotic-Assisted Versus Thoracoscopic Lobectomy Outcomes From High-Volume Thoracic Surgeons. Ann Thorac Surg 2018;106:902-8. [Crossref] [PubMed]
- Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187-96. [Crossref] [PubMed]
- Paradela M, Garcia-Perez A, Fernandez-Prado R, et al. Uniportal robotic versus thoracoscopic assisted surgery: a propensity score-matched analysis of the initial 100 cases. Ann Cardiothorac Surg 2023;12:23-33. [Crossref] [PubMed]
- Jonas DE, Reuland DS, Reddy SM, et al. Screening for Lung Cancer With Low-Dose Computed Tomography: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2021;325:971-87. [Crossref] [PubMed]
- Wang L, Ge L, You S, et al. Lobectomy versus segmentectomy in patients with stage T (> 2 cm and ≤ 3 cm) N0M0 non-small cell lung cancer: a propensity score matching study. J Cardiothorac Surg 2022;17:110. [Crossref] [PubMed]
- Harada H, Okada M, Sakamoto T, et al. Functional advantage after radical segmentectomy versus lobectomy for lung cancer. Ann Thorac Surg 2005;80:2041-5. [Crossref] [PubMed]
- Altorki N, Wang X, Kozono D, et al. Lobar or Sublobar Resection for Peripheral Stage IA Non-Small-Cell Lung Cancer. N Engl J Med 2023;388:489-98. [Crossref] [PubMed]
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, andomized, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
- Cerfolio RJ, Watson C, Minnich DJ, et al. One Hundred Planned Robotic Segmentectomies: Early Results, Technical Details, and Preferred Port Placement. Ann Thorac Surg 2016;101:1089-95; Discussion 1095-6. [Crossref] [PubMed]
- Gergen AK, White AM, Mitchell JD, et al. Introduction of robotic surgery leads to increased rate of segmentectomy in patients with lung cancer. J Thorac Dis 2021;13:762-7. [Crossref] [PubMed]
- Zhou N, Corsini EM, Antonoff MB, et al. Robotic Surgery and Anatomic Segmentectomy: An Analysis of Trends, Patient Selection, and Outcomes. Ann Thorac Surg 2022;113:975-83. [Crossref] [PubMed]
- Kocher G, Deckarm S, Flury D. Completely portal robotic Pancoast tumour resection with en bloc resection of the left upper lobe and chest wall. Multimed Man Cardiothorac Surg 2023;2023: [Crossref] [PubMed]
- Flury DV, Diezi M, Lutz JA, et al. Uniportal VATS and hybrid VATS en bloc lung and chest wall resection—report of surgical technique and own experience. Video-assist Thorac Surg 2023;8:45. [Crossref]
- Manolache V, Motas N, Bosinceanu ML, et al. Comparison of uniportal robotic-assisted thoracic surgery pulmonary anatomic resections with multiport robotic-assisted thoracic surgery: a multicenter study of the European experience. Ann Cardiothorac Surg 2023;12:102-9. [Crossref] [PubMed]
- Sharma R. Mapping of global, regional and national incidence, mortality and mortality-to-incidence ratio of lung cancer in 2020 and 2050. Int J Clin Oncol 2022;27:665-75. [Crossref] [PubMed]
- Ganti AK, Klein AB, Cotarla I, et al. Update of Incidence, Prevalence, Survival, and Initial Treatment in Patients With Non-Small Cell Lung Cancer in the US. JAMA Oncol 2021;7:1824-32. [Crossref] [PubMed]
- Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243-60. [Crossref] [PubMed]
- Heist RS, Mino-Kenudson M, Sequist LV, et al. FGFR1 amplification in squamous cell carcinoma of the lung. J Thorac Oncol 2012;7:1775-80. [Crossref] [PubMed]
- Li C, Lu H. Adenosquamous carcinoma of the lung. Onco Targets Ther 2018;11:4829-35. [Crossref] [PubMed]
- Tseng CH, Chen KC, Hsu KH, et al. EGFR mutation and lobar location of lung adenocarcinoma. Carcinogenesis 2016;37:157-62. [Crossref] [PubMed]
- Kinsey CM, Estepar RS, Zhao Y, et al. Invasive adenocarcinoma of the lung is associated with the upper lung regions. Lung Cancer 2014;84:145-50. [Crossref] [PubMed]
- Yang S, Guo W, Chen X, et al. Early outcomes of robotic versus uniportal video-assisted thoracic surgery for lung cancer: a propensity score-matched study. Eur J Cardiothorac Surg 2018;53:348-52. [Crossref] [PubMed]
- Novellis P, Bottoni E, Voulaz E, et al. Robotic surgery, video-assisted thoracic surgery, and open surgery for early stage lung cancer: comparison of costs and outcomes at a single institute. J Thorac Dis 2018;10:790-8. [Crossref] [PubMed]
- Toker A, Özyurtkan MO, Demirhan Ö, et al. Lymph Node Dissection in Surgery for Lung Cancer: Comparison of Open vs. Video-Assisted vs. Robotic-Assisted Approaches. Ann Thorac Cardiovasc Surg 2016;22:284-90. [Crossref] [PubMed]
- Veronesi G, Abbas AE, Muriana P, et al. Perioperative Outcome of Robotic Approach Versus Manual Videothoracoscopic Major Resection in Patients Affected by Early Lung Cancer: Results of a Randomized Multicentric Study (ROMAN Study). Front Oncol 2021;11:726408. [Crossref] [PubMed]
- Rocha Júnior E, Terra RM. Robotic lung resection: a narrative review of the current role on primary lung cancer treatment. J Thorac Dis 2022;14:5039-55. [Crossref] [PubMed]
- Zhang Y, Chen C, Hu J, et al. Early outcomes of robotic versus thoracoscopic segmentectomy for early-stage lung cancer: A multi-institutional propensity score-matched analysis. J Thorac Cardiovasc Surg 2020;160:1363-72. [Crossref] [PubMed]
- Vansteenkiste J, De Ruysscher D, Eberhardt WE, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24:vi89-98. [Crossref] [PubMed]
- Manser R, Wright G, Hart D, et al. Surgery for early stage non-small cell lung cancer. Cochrane Database Syst Rev 2005;2005:CD004699. [PubMed]
- Allen MS, Darling GE, Pechet TT, et al. Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg 2006;81:1013-9; discussion 1019-20. [Crossref] [PubMed]
- Darling GE, Allen MS, Decker PA, et al. Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in the patient with N0 or N1 (less than hilar) non-small cell carcinoma: results of the American College of Surgery Oncology Group Z0030 Trial. J Thorac Cardiovasc Surg 2011;141:662-70. [Crossref] [PubMed]
- Deen SA, Wilson JL, Wilshire CL, et al. Defining the cost of care for lobectomy and segmentectomy: a comparison of open, video-assisted thoracoscopic, and robotic approaches. Ann Thorac Surg 2014;97:1000-7. [Crossref] [PubMed]
- Keeney-Bonthrone TP, Frydrych LM, Karmakar M, et al. Robot-assisted vs. video-assisted thoracoscopic lobectomy: a systematic review of cost effectiveness. Video-assist Thorac Surg 2020;5:4. [Crossref]
- Kneuertz PJ, Singer E, D’Souza DM, et al. Hospital cost and clinical effectiveness of robotic-assisted versus video-assisted thoracoscopic and open lobectomy: A propensity score-weighted comparison. J Thorac Cardiovasc Surg 2019;157:2018-2026.e2. [Crossref] [PubMed]
- Swanson SJ, Miller DL, McKenna RJ Jr, et al. Comparing robot-assisted thoracic surgical lobectomy with conventional video-assisted thoracic surgical lobectomy and wedge resection: results from a multihospital database (Premier). J Thorac Cardiovasc Surg 2014;147:929-37. [Crossref] [PubMed]
- Worrell SG, Dedhia P, Gilbert C, et al. The cost and quality of life outcomes in developing a robotic lobectomy program. J Robot Surg 2019;13:239-43. [Crossref] [PubMed]
Cite this article as: Kocher GJ, Wegener S, Deckarm S, Flury DV. Uniportal & biportal robotic anatomic lung resection (without CO2 insufflation): technique, initial experience and cost. J Vis Surg 2024;10:6.