
Robotic transduodenal ampullectomy: an alternative surgical technique for benign tumors at the ampulla of Vater
Highlight box
Key findings
• Submucosal injection of Eleview® blue viscous solution for dissection and resection of a benign tumor at the ampulla of Vater.
• Robotic approach for resection of a benign tumor at the ampulla of Vater compared to the historic open approach.
What is conventional and what is novel/modified?
• Transduodenal ampullectomy via open approach is conventional.
• Transduodenal ampullectomy via the robotic approach and via the use of submucosal injection of Eleview® blue viscous solution for dissection and resection of a tumor at the ampulla of Vater are novel.
What is the implication, and what should change now?
• All benign tumors at the ampulla of Vater can be considered resectable via this study’s approach.
Introduction
Background
Periampullary tumors are rare lesions that originate in a complex anatomical region including the ampulla of Vater, distal common bile duct (CBD), second portion of the duodenum, head of pancreas, and pancreatic duct (PD) (1). Tumors of the ampulla of Vater are distinct from cancers of the duodenum, CBD, and pancreas in that they are distal to the confluence of the CBD and PD (2). These lesions may be benign or malignant, and the mainstay treatment is excision (3,4). Options for excision have historically included pancreaticoduodenectomy, open transduodenal resection, and endoscopic resection (5).
Rationale
One of the main difficulties guiding treatment according to histology is that there is not always microscopic pathology prior to surgical or endoscopic excision (6). Even when tissue samples are obtained, they often do not reveal an occult malignancy, especially if the lesion is too large to completely resect endoscopically. When malignancy is suspected or confirmed, pancreatoduodenectomy remains paramount in treatment (7,8). However, when benign pathology can be established during the preoperative workup, endoscopic or transduodenal ampullectomy can be considered (9). For lesions that are unable to be completely resected endoscopically, transduodenal ampullectomy has traditionally been performed via an open approach, and as early as 2003 the procedure has been performed via the laparoscopic approach (10,11). However, adoption of minimally invasive transduodenal ampullectomy via laparoscopy remained limited due to the complex surgical anatomy of the ampulla.
Objective
With recent advancements of minimally invasive surgery via robotics, the technical challenges due to complex surgical anatomy could be mitigated. Specifically, the dissection of the complex anatomical region including the ampulla of Vater, CBD, second portion of the duodenum, head of pancreas, and PD via laparoscopy or open approach can be mitigated via the wristed instruments and camera of the robot. This paper presents a novel approach of robotic transduodenal ampullectomy as an acceptable treatment option for benign tumors at the ampulla of Vater using submucosal injection of Eleview® blue viscous solution (Video 1). The video provides step-by-step instruction on the preparation, robotic access and visualization, dissection, Eleview® blue viscous solution injection, and resection of a benign ampullary tumor. We present this article in accordance with the SUPER reporting checklist (available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-21/rc).
Preoperative preparations and requirements
Patient selection and workup
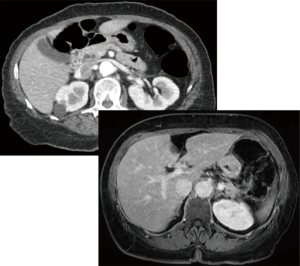
A 68-year-old female presented with early satiety, significant weight loss, and inability to tolerate oral intake across 3 months. Computerized tomography (CT) showed a double duct sign as seen in Figure 1. Subsequent magnetic resonance imaging (MRI) showed an equivocal ampullary lesion. The patient then underwent an esophagogastroduodenoscopy (EGD) with endoscopic ultrasonography (EUS) in which an ampullary mass was identified. A biopsy was performed and was consistent with a low-grade neuroendocrine tumor. Due to bile duct extension noted at the time of EGD with EUS, endoscopic resection was deemed to be an increased risk for positive margin, perforation, and/or ductal injury. Further treatment options were discussed with the patient; these options included an open resection that would result in higher morbidity and hospital length of stay versus endoscopic resection would result in higher risk of perforation given the location of the lesion and involvement of the bile duct and PD. Ultimately, a robotic transduodenal ampullectomy was the operation of choice.
Equipment preference card
All robotic cases are performed using the da Vinci Xi® robotic platform (Intuitive Surgical, Sunnyvale, CA, USA). Robotic instruments include monopolar curved scissors, fenestrated bipolar forceps, a vessel sealer device, and PrograspTM forceps. The large needle driver is used for suturing. Monocryl® and V-LocTM sutures (Medtronic, Minneapolis, MN, USA) are used for anastomoses and closure of the duodenum, respectively.
Surgical team
A surgeon with previous robotic hepatopancreaticobiliary experience is necessary to lead the surgical team. An anesthesiologist, an operating room nurse, and a scrub technician who also have experience in robotic hepatopancreaticobiliary surgery are necessary for a successful operation.
Ethical standard
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript, the accompanying image, and the video. A copy of the written consent is available for review by the editorial office of this journal.
Step-by-step description of surgical technique
The patient is taken to the operating room and placed in the supine position. General anesthesia is induced, and an arterial line and two peripheral intravenous lines are placed. A nasogastric tube and urinary catheter are placed along with sequential compression devices on the patient’s lower extremities. Both arms are extended out on arm boards for anesthesia access. Piperacillin/tazobactam is the perioperative antibiotic of choice when manipulating the biliary system.
Pneumoperitoneum is obtained with a Veress needle in an infraumbilical incision. An initial 8 mm robotic trocar is placed and laparoscopy is performed to confirm no evidence of metastasis. The patient is then placed into reverse Trendelenburg position for approximately 8–10 degrees. Four 8 mm robotic trocars are placed across the upper abdomen in a straight line, and the infra-umbilical port is changed to a 12 mm laparoscopic port for assistance. The robot is then docked, and the robotic arms and instruments are connected. Arm 1 carries the fenestrated bipolar and arm 2 is reserved for the robotic camera. Arm 4 carries the PrograspTM for retraction and arm 3 is used as the “working port” where all instrument exchanges occur.
The round ligament is first harvested from the anterior abdominal wall. The gallbladder is suspended to the abdominal wall with a suture to aid in liver retraction. Retraction of the left lateral sector is achieved by placing sponges under the liver. Initial dissection of the upper abdomen is performed by mobilizing the greater curvature of the stomach and entering the lesser sac with a combination of monopolar scissors and vessel sealing device. The dissection is continued medial over the face of the pancreas and mobilizing the transverse colon mesentery and hepatic flexure of the colon down to expose the right kidney and the duodenum. A Kocher maneuver is then performed to where the second portion of the duodenum is exposed.
Intraoperative ultrasound is then utilized to assess vascular and ductal anatomy in relation to the ampullary tumor. A longitudinal incision is made along the second portion of the duodenum. The ampullary tumor is easily identified as a firm mass. If preoperative CBD or PD stents were placed, these will be seen at this time and can be removed. A suture is used to suspend the ampullary mass away from the duodenal wall. Eleview® blue viscous solution is injected into the submucosa circumferentially using a Carr-Locke injection needle to exaggerate this plane. Monopolar scissors with cutting current are used to open the mucosa and further develop the submucosal plane around the ampullary mass. It should be noted at this time whether the mass is involving the CBD and/or PD. When involved, the mass is amputated to include the distal portions of the ducts using the robotic monopolar scissors with cutting current. In cases of suspected malignancy, once the ampulla is excised it will be sent for frozen pathologic evaluation. If margins are positive or invasive cancer is present, the procedure is converted to a pancreaticoduodenectomy. If no invasive cancer is present, the distal CBD and PD are then reimplanted into the duodenal mucosa using interrupted 5-0 and 6-0 Monocryl sutures. Stents are placed across both the PD and CBD anastomoses. Two 4-0 V-LocTM sutures are then used to re-approximate and close the duodenotomy. The round ligament harvested at the beginning of the case is then used to cover the duodenal suture line. We prefer to use a fibrin glue sealant over the round ligament flap and a drain is placed near the repair.
The gallbladder is removed, and we use flowable hemostatic agents for hemostasis. The 15 mm incision is closed with interrupted suture. The fascia of the 8 mm trocar sites is not closed. Incisions are closed with 4-0 MonocrylTM. Operative time is approximately 120 minutes. Blood loss is minimal at 50 mL.
Postoperative considerations and tasks
An upper gastrointestinal (UGI) series is obtained on postoperative day one. In this patient, the UGI showed an intact duodenal repair without obstruction or leak. The patient’s diet was slowly advanced upon return of bowel function. This patient had an uneventful hospital course and was discharged on post-operative day 4. Final pathology for this patient showed a 1.3 cm grade I ampullary neuroendocrine tumor resected with negative margins.
Tips and pearls
- A 12 mm assistant port is essential for passing suture and sponges as well as suctioning.
- Place sponges under the liver to aid in retraction of the left lobe and exposure. This will obviate the need for additional liver retractors.
- The round ligament can be harvested as a well vascularized flap to cover the duodenotomy repair.
- Always use intraoperative ultrasound to identify vascular and ductal anatomy in comparison to the ampullary mass.
- If the ampulla is difficult to find, the cystic duct can be cannulated with a catheter and fed distal through the bile duct towards the ampulla.
- Injection of Eleview® blue viscous solution helps expose the planes for dissection of the ampulla.
Discussion
Surgical highlights
The surgical highlights from this paper include performing transduodenal ampullectomy via the robotic approach using submucosal injection of Eleview® blue viscous solution for the dissection and resection of a benign tumor at the ampulla of Vater.
Strengths and limitations
This paper provides an alternative approach to difficult to access benign tumors of the ampulla of Vater without needing to convert to an open procedure or perform a more invasive surgery such as a pancreaticoduodenectomy. Limitations of this paper are that a surgeon needs to have experience with pancreas resections from an open and robotic perspective prior to attempting this technique. Furthermore, some institutions may not have robotic access available to them, limiting their ability to exercise this technique.
Comparisons with other surgical techniques and research
In comparison with an open and laparoscopic approaches, exposure, visualization, dissection, resection, and reconstruction are technically less difficult, and patients recover more quickly with the robotic approach. Furthermore, the robotic approach offers greater precision, less pain, and shorter recovery times as previously reported in the literature.
Implications and actions recommended
Future studies are necessary to compare the outcomes and benefits to this robotic approach compared to laparoscopic and open approaches.
Conclusions
We describe our technique for minimally invasive transduodenal ampullectomy using the robotic platform for the treatment of a low-grade neuroendocrine tumor at the ampulla of Vater. This method, which utilizes a submucosal injection of Eleview® blue viscous solution, allows for precise dissection of the ampulla at the level of the distal CBD and PD. The robotic platform is an ideal tool for this procedure due to the three-dimensional camera and wristed instruments allowing us to easily re-implant the CBD and PD in a minimally invasive fashion. With this technique, morbid procedures such as a pancreaticoduodenectomy or open resection may be able to be avoided for benign disease.
Acknowledgments
The manuscript has been presented at Podium presentation at the Clinical Robotic Surgery Association (CRSA) World Congress in Rome, Italy, December 2022.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the SUPER reporting checklist. Available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-21/rc
Peer Review File: Available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-21/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-21/coif). J.B.M. and D.V. are consultants for Intuitive Surgical. F.N.M.’s fellowship stipend is sponsored by Intuitive Surgical. The other author has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript, the accompanying image and the video. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Taliente F, Bianco G, Moschetta G, et al. From endoscopic resection to pancreatoduodenectomy: a narrative review of treatment modalities for the tumors of the ampulla of Vater. Chin Clin Oncol 2022;11:23. [Crossref] [PubMed]
- Howe JR, Klimstra DS, Moccia RD, et al. Factors predictive of survival in ampullary carcinoma. Ann Surg 1998;228:87-94. [Crossref] [PubMed]
- El Hajj II, Coté GA. Endoscopic diagnosis and management of ampullary lesions. Gastrointest Endosc Clin N Am 2013;23:95-109. [Crossref] [PubMed]
- Albores-Saavedra J, Schwartz AM, Batich K, et al. Cancers of the ampulla of vater: demographics, morphology, and survival based on 5,625 cases from the SEER program. J Surg Oncol 2009;100:598-605. [Crossref] [PubMed]
- Heise C, Abou Ali E, Hasenclever D, et al. Systematic Review with Meta-Analysis: Endoscopic and Surgical Resection for Ampullary Lesions. J Clin Med 2020;9:3622. [Crossref] [PubMed]
- Panzeri F, Crippa S, Castelli P, et al. Management of ampullary neoplasms: A tailored approach between endoscopy and surgery. World J Gastroenterol 2015;21:7970-87. [Crossref] [PubMed]
- Bassi C, Marchegiani G, Giuliani T, et al. Pancreatoduodenectomy at the Verona Pancreas Institute: the Evolution of Indications, Surgical Techniques, and Outcomes: A Retrospective Analysis of 3000 Consecutive Cases. Ann Surg 2022;276:1029-38. [Crossref] [PubMed]
- Network NCC. Ampullary Adenocarcinoma. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). 2023; Version 1.2023.
- Jung YK, Paik SS, Choi D, et al. Transduodenal ampullectomy for ampullary tumor. Asian J Surg 2021;44:723-9. [Crossref] [PubMed]
- Halsted WS. Contributions to the surgery of the bile passages, especially of the common bile-duct. Boston Med Surg J 1899;131:645-54. [Crossref]
- Rosen M, Zuccaro G, Brody F. Laparoscopic resection of a periampullary villous adenoma. Surg Endosc 2003;17:1322-3. [Crossref] [PubMed]
Cite this article as: Ricker AB, McCarron FN, Vrochides D, Martinie JB. Robotic transduodenal ampullectomy: an alternative surgical technique for benign tumors at the ampulla of Vater. J Vis Surg 2024;10:1.


