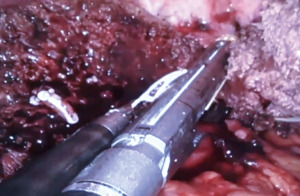
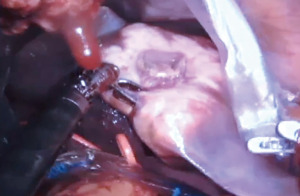
Robotic left hepatectomy for colorectal liver metastases with modified rubber-band technique: surgical technique & steps
Highlight box
Surgical highlights
• Left hepatectomy—resection of segments II, III, IV (transection line-cantlie line/principal plane).
• Minimally-invasive—Da Vinci Xi Robotic platform with laparoscopic assistance.
• Minimal blood-loss, reduced hospital stay, oncological safety and acceptable outcomes.
What is conventional and what is novel/modified?
• Conventionally, robotic hepatectomy is prohibitive due to cost implications.
• Only four robotic instruments used i.e., Maryland bipolar, monopolar diathermy, ProGrasp forceps and hemolock applicator.
• Modified Rubber-band technique. The original technique used elastic rubber bands on either side of the transection plane. Here, we use silicon vessel tapes which are more durable and provide graded traction.
• Laparoscopic instruments for assistance.
What is the implication, and what should change now?
• Four robotic instruments reduce procedural cost.
• Transection plane is in line with the camera (R2 port) providing better visualization aiding effective control, minimizing blood loss. Graded traction provides consistent & uniform pull.
Introduction
Background
Colorectal liver metastases (CRLMs) are seen in ~25–30% of patients worldwide with surgical resection being curative in selected patients. Incidence of synchronous CRLM is ~13.8–17.1% while that for metachronous CRLM is ~7.6–15.1% (1). Liver resections have had higher operative mortality, upwards of 20% in the 1980s, compared to other organ resections (2). With better understanding of the anatomy of the liver, improved operative techniques, better instruments, and perioperative anesthesia care, operative mortality in most tertiary care centers is now under 5% (3,4). Open liver resections (OLRs) have been the norm traditionally. With the advent of minimally invasive surgery (MIS), laparoscopic liver resections (LLRs) and robotic liver resections (RLRs) are finding greater utility due to obvious advantages. A meta-analysis comparing open and laparoscopic CRLM resection found no significant differences in long-term oncologic outcomes (5). The robotic platform provides significant ergonomic advantages over laparoscopy with a greater range of motion with articulating instruments, better three-dimensional vision and a stable camera. Retrospective studies comparing laparoscopy with the robotic approach, however, do indicate longer operative times, inconsistently a higher blood loss and increased cost although perioperative and short-term outcomes seem comparable (6). However, interest in robotic liver surgeries has peaked in recent times and with promising results, the robotic approach seems to be eminently feasible and a safe alternative in liver surgeries (7).
Parenchymal transection seems to be the ‘Achilles heel’ of MIS liver resections with the resulting higher blood loss and longer operative times. The rubber-band technique popularized by Choi et al. (8), seems to be an effective technique for this step which has been employed in our video with a slight modification of using silicon vessel tapes. This technique brings the transection plane in line with the camera (R2 port) providing better visualization of structures crossing the principal plane thereby aiding effective control of vessels and minimizing blood loss. Graded traction provides consistent pull even up to the upper part of transection.
Rationale
One of the major impediments to minimally invasive hepatic resections has been the difficulty in managing hemorrhage, leading to a longer learning curve and reluctance to pursue laparoscopic resections. This is reflected in the international consensus statements of Louisville 2009 (9) and Morioka 2015 (10). However, with increasing experience, surgeons have expanded indications for minimally invasive hepatectomies.
At our institution, OLR has been the norm with carefully selected patients subjected to the robotic platform. We started RLRs in 2015 and our initial experience has had acceptable outcomes. Out of a total of 31 robotic hepatectomies, 67.7% were left lateral sectionectomies, with about 10% major hepatectomies (right and left), 10% non-anatomical resections and the rest were robotic-assisted resections.
Objective
In this article, we outline our technique of minimally invasive left hepatectomy using the Da Vinci Xi Robotic surgical system (Intuitive Surgical, Sunnyvale, CA, USA) with laparoscopic ports for assistance (hybrid method), thereby reducing the number of robotic instruments utilized. We present this article in accordance with the SUPER reporting checklist (available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-23/rc).
Preoperative preparations and requirements
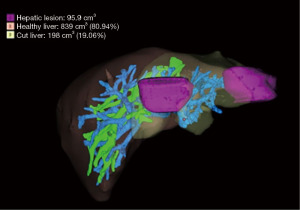
Our patient is a 58-year-old gentleman, with good performance and no medical comorbidities, diagnosed with synchronous hepatic metastases from a rectal adenocarcinoma in segments II and IVa. The patient received long-course neoadjuvant chemoradiation followed by a laparoscopic abdominoperineal resection for a cT3N1 rectal tumour. One month after the index surgery, he was planned for a left hepatectomy using the robotic platform (Da Vinci Xi) after confirmation of an adequate functional liver remnant (FLR) of 80.9% on a Myrian protocol contrast-enhanced computed tomography scan as a staged approach (Figure 1). The alpha fetoprotein level was normal & carcinoembryonic antigen (CEA) level was 2.9 ng/mL. The patient received adjuvant capecitabine plus oxaliplatin (CapOX) chemotherapy after hepatectomy and is on regular follow-up and doing well. Informed consent was obtained from the patient for recording the surgery.
Our current criteria for selecting patients for a RLR in CRLM include solitary or multiple lesions in the same lobe, 5 cm or less in size, in segments II, III, IV, V or VI as per the Louisville consensus statement.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript, any accompanying images and the video. A copy of the written consent is available for review by the editorial office of this journal.
Step-by-step description (Video 1)
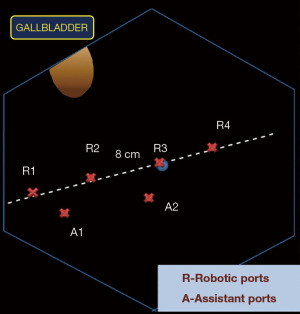
Step 1: port placement
Our standard port placement for RLRs includes four robotic and two assistant ports. Robotic ports are inserted 8 cm apart on an oblique line running from the right iliac fossa along the umbilicus to the left hypochondrium. Assistant ports are placed 4 cm below this line between the R1–R2 and the R2–R3 ports as shown in the schematic (Figure 2).
Step 2: dissection of the porta hepatis
Diagnostic laparoscopy is done to rule out disseminated disease.
- Cholecystectomy. The Calot’s triangle is dissected to delineate the cystic artery, and the cystic duct, which are clipped and ligated to complete the cholecystectomy.
- Inflow control. The liver is retracted upwards and the porta is dissected to delineate all major structures viz., the common bile duct, common hepatic artery, and the portal vein (Figure 3). The left hepatic artery (LHA) is dissected, ligated, and divided, revealing the left branch of the portal vein (LPV). A vascular clamp is applied over the left portal vein and the liver traction is released. A demarcation line seen over the anterior surface of the liver is marked with diathermy. The LPV is divided with a vascular stapler. This completes the division of vascular inflow to the left liver.
- Bile duct division. We prefer to divide the left hepatic duct intra-parenchymally, however in this case, due to a long extra-hepatic course it was clipped and divided before parenchymal transection.

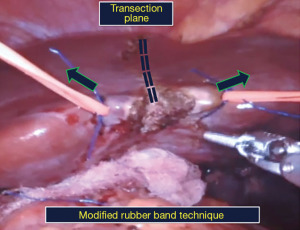
Step 3: liver mobilization and parenchymal transection (modified rubber band technique)
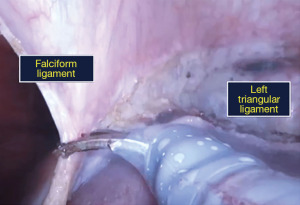
- Peritoneal attachments of the left liver viz., the falciform ligament, and anterior and posterior folds of the left triangular ligament are divided (Figure 4).
Parenchymal transection. We use a modification of the rubber band technique popularized by Choi et al. (8). Two vessel tapes are sutured to the liver on either side of the transection plane which are pulled up, to bring the transection plane in line with the camera inserted through the R2 port (Figure 5). Parenchymal transection is undertaken with a combination of monopolar diathermy scissors and bipolar forceps, preserving the middle hepatic vein (Figure 6). The left hepatic vein is divided using a vascular stapler (Figure 7).
Any leaking bile ducts are clipped, meticulous hemostasis is achieved and an absorbable hemostatic patch is placed over the cut surface of liver. The specimen is retrieved (Figure 8) via a specimen bag by connecting two of the robotic port sites or via a small suprapubic transverse incision.
Our patient had a blood loss of 600 cc, a specimen weight of 420 g and the operative duration was 340 min.
Postoperative considerations and tasks
Meticulous postoperative management forms the cornerstone of effective therapy after a formal hepatectomy with steps to prevent the development of post-hepatectomy liver failure (PHLF) which begins with due consideration for the adequacy of the FLR as assessed by the preoperative Myrian protocol scan. The CRLM resection consensus guidelines [2006] recommend the acceptable FLR to be >20% of total liver volume (TLV) in normal livers, >30% in the presence of steatosis and >40% in the presence of fibrosis/cirrhosis (11). Prevention strategies and management of PHLF is a complex undertaking with the below tables depicting the main principles (12) (Table 1, Figure 9).
Table 1
| Surgical strategies |
| • Adequacy of functional liver remnant preoperatively |
| • Portal vein embolization |
| • Ischemic preconditioning |
| • Two-stage hepatectomy |
| • In-situ hypothermic liver perfusion |
| • Portal ligation and in-situ splitting |
| Pharmacological—role of somatostatin |
| Preoperative preparation with management of comorbidities and nutritional optimization |
| Meticulous surgical and anesthetic measures—minimizing blood loss and transfusion, low central venous pressure during parenchymal transection, minimizing operative duration |
| Vigilance for postoperative issues like biliary leaks and hemorrhage |
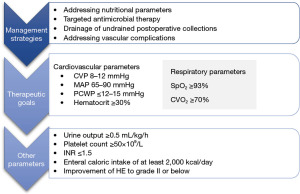
Significant hemorrhage with hemodynamic instability and undrained perihepatic collections in the postoperative period might require a low threshold for re-interventions depending on the clinical situation.
Tips and pearls
- Preoperative assessment of the adequacy of FLR.
- Proper assessment and recognition of anatomical variations before ligation of the inflow system to the specimen. Ligation of LHA after confirmation of intact right hepatic artery (RHA) pulsation upon clamping, awareness about the segment IV branch arising occasionally from the RHA, preservation of the occasional caudate lobe portal vein branch arising from the LPV by ligating the LPV between the caudate branch and the insertion of ligamentum venosum, awareness of right sectoral biliary ducts draining into the left hepatic duct are some commonly encountered variations to be aware of.
- Rubber-band technique for ergonomic parenchymal transection. One of the major challenges in MIS liver resections is the spatial relationship of the three-dimensional liver anatomy and the limited range of motion and fulcrum effect of the rigid instruments especially in laparoscopy. Therefore, successful outcomes can be achieved by aligning the transection plane with the camera so that hemostasis, biliostasis and tumour-free margins can be ensured. The rubber-band technique is a good approach in this direction as it exposes the transection plane and the structures crossing it for easy access and durable control improving the procedural ergonomics.
- Additional laparoscopic ports for assistance while reducing the number of robotic instruments to reduce overall procedural cost.
- Specimen retrieval through small incision by joining two robotic ports or a suprapubic Pfannenstiel incision. Retrieval of the specimen through either of these incisions is feasible. The Pfannenstiel incision is preferred due to obvious advantages of reducing postoperative pain and improved respiratory mechanics.
- Ensuring good hemostasis and biliostasis. One of the significant causes of PHLF is blood loss >1,200 mL and consequent intraoperative transfusions (12). Meticulous hemostasis with clips and ligatures helps mitigate these issues. Meticulous biliostasis can avoid infective complications and interventions in the post-operative period.
Discussion
Surgical highlights
With this surgical video demonstration, we intend to demonstrate the technique and tips for a safe robotic left hepatectomy. The international consensus statement of 2009 at Louisville recommended the use of minimally invasive liver resections in patients with solitary lesions, 5 cm or less, located in liver segments 2 to 6 with laparoscopic left lateral sectionectomy considered standard practice (9). The statement also concluded that major hepatectomies (right or left), although feasible by the minimally invasive approach, should be reserved for surgeons with greater experience, facile with advanced laparoscopic techniques.
Over the next 6 years, a significant increase in the acceptability of LLRs resulted in an additional 9,000 resections leading to the second international consensus conference held in Morioka, in 2015. This consensus conference evaluated the role of LLRs with the available evidence and concluded that minor LLRs had become standard practice and major LLRs were still innovative procedures in the exploration phase. The statement encouraged the continued and cautious introduction of major LLRs into practice and the generation of higher-quality evidence to evaluate the utility and outcomes (10).
The international consensus statement on RLRs in 2018 concluded that hepatectomy is associated with longer operative time, possibly higher blood loss, and greater cost. In malignancy, robotic hepatectomy had similar effectiveness as compared to open and laparoscopic hepatectomy without a significant difference in the resection rates, overall survival (OS), or recurrence (13).
Strengths and limitations
The robotic platform provides important advantages over the conventional laparoscopic platform with a stable camera, greater degrees of freedom, motion scaling, and tremor filtration. This could translate into a higher number of cases being completed without conversion to hybrid or hand-assisted procedures. A recent study by Tsung et al., which compared single-institution laparoscopic and robotic hepatectomies, noted that over 90% of the robotic hepatectomies did not need conversion while only 49.1% were accomplished without conversion when conventional laparoscopy was used (14). Intraoperative uncontrolled hemorrhage is one of the predominant indications for conversion while an inability to achieve an R0 resection is the next (15). The intermittent Pringle maneuver can be an effective method of reducing intraoperative hemorrhage in minimally invasive hepatectomy. We use this maneuver selectively using the Huang Loop technique (not utilized in this case) (16).
Robotic liver surgery has significant cost implications, especially in low and middle-income countries, which has been prohibitive to widespread application. In addition, with the perennial focus on value-driven healthcare and cost-cutting, several authors have questioned the need for the robotic platform over standard laparoscopic surgery (17). Other authors have argued that savings due to fewer complications and shorter hospital stays seem to offset the higher cost. We were able to reduce the cost of the procedure with the use of only four robotic instruments i.e., Maryland bipolar forceps, monopolar diathermy scissors, ProGrasp forceps and robotic hemolock applicator. Utilizing the laparoscopic port for stapling further reduced the overall cost of the procedure vis-à-vis using a robotic stapler. Our experience with using the robotic platform, especially for liver resections is still in its infancy for drawing credible conclusions regarding its cost implications. With the accumulation of more experience and a larger sample size, conclusive data could be generated.
Comparison with other surgical techniques and researches
Several series from western countries and Japan have reported robust outcomes with the use of LLR for CRLM. Most of these studies have compared outcomes with OLRs and found fewer postoperative complications (18) while having similar oncologic outcomes (updated results of the OSLO-COMET trial) (19). Studies comparing robotic and LLRs inconsistently note longer operative times with the robotic approach but with lower blood loss and readmission rates (20) although long-term oncologic outcomes were similar (6). Other advantages of the robotic platform include the ease of addressing lesions in the posterosuperior segments of the liver which were difficult to access using a purely laparoscopic approach. A recent multicenter propensity score matching analysis compared long-term outcomes of RLR and LLR and noted similar perioperative outcomes and long-term oncologic outcomes [OS 61% vs. 60%, P=0.78, and disease-free survival (DFS) 38% vs. 44%, P=0.62, postmatching] (6).
Implications and actions recommended
Literature suggests comparable oncologic outcomes of MIS versus OLRs albeit with significant short-term advantages. Outcomes of LLRs and RLRs were also similar, cost being a prohibitive factor for widespread use of RLRs although the robotic platform does provide significant ergonomic advantages. With widespread use, demonstrated safety and feasibility, RLRs are here to stay and are an important technical advancement in MIS liver resections.
Conclusions
Hepatectomy is feasible by the minimally invasive technique at high-volume tertiary care centres in experienced hands providing good outcomes. Patient selection is key. Improved ergonomics provided by the robotic platform deliver good operative outcomes. Cost implications of the robotic platform are a significant factor preventing widespread use.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the SUPER reporting checklist. Available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-23/rc
Peer Review File: Available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-23/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.com/article/view/10.21037/jovs-23-23/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript, any accompanying images and the video. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Pagani M, De Vincenti R, Cecchi C, et al. Hepatic Resection in Patients with Colo-Rectal Liver Metastases: Surgical Outcomes and Prognostic Factors of Single-Center Experience. J Clin Med 2023;12:2170. [Crossref] [PubMed]
- Fortner JG, Blumgart LH. A historic perspective of liver surgery for tumors at the end of the millennium. J Am Coll Surg 2001;193:210-22. [Crossref] [PubMed]
- Jarnagin WR, Gonen M, Fong Y, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg 2002;236:397-406; discussion 406-7. [Crossref] [PubMed]
- Patkar S, Parray A, Kanetkar A, et al. Towards Standardization of Liver Resections in India: Five Hundred Consecutive Oncological Liver Resections- Trends, Techniques and Outcomes. J Gastrointest Cancer 2021;52:651-8. [Crossref] [PubMed]
- Xie SM, Xiong JJ, Liu XT, et al. Laparoscopic Versus Open Liver Resection for Colorectal Liver Metastases: A Comprehensive Systematic Review and Meta-analysis. Sci Rep 2017;7:1012. [Crossref] [PubMed]
- Beard RE, Khan S, Troisi RI, et al. Long-Term and Oncologic Outcomes of Robotic Versus Laparoscopic Liver Resection for Metastatic Colorectal Cancer: A Multicenter, Propensity Score Matching Analysis. World J Surg 2020;44:887-95. [Crossref] [PubMed]
- Schmelzle M, Feldbrügge L, Ortiz Galindo SA, et al. Robotic vs. laparoscopic liver surgery: a single-center analysis of 600 consecutive patients in 6 years. Surg Endosc 2022;36:5854-62. [Crossref] [PubMed]
- Choi SH, Choi GH, Han DH, et al. Laparoscopic liver resection using a rubber band retraction technique: usefulness and perioperative outcome in 100 consecutive cases. Surg Endosc 2015;29:387-97. [Crossref] [PubMed]
- Buell JF, Cherqui D, Geller DA, et al. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg 2009;250:825-30. [Crossref] [PubMed]
- Wakabayashi G, Cherqui D, Geller DA, et al. Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 2015;261:619-29. [PubMed]
- Abdalla EK, Adam R, Bilchik AJ, et al. Improving resectability of hepatic colorectal metastases: expert consensus statement. Ann Surg Oncol 2006;13:1271-80. [Crossref] [PubMed]
- Ray S, Mehta NN, Golhar A, et al. Post hepatectomy liver failure - A comprehensive review of current concepts and controversies. Ann Med Surg (Lond) 2018;34:4-10. [Crossref] [PubMed]
- Liu R, Wakabayashi G, Kim HJ, et al. International consensus statement on robotic hepatectomy surgery in 2018. World J Gastroenterol 2019;25:1432-44. [Crossref] [PubMed]
- Tsung A, Geller DA, Sukato DC, et al. Robotic versus laparoscopic hepatectomy: a matched comparison. Ann Surg 2014;259:549-55. [Crossref] [PubMed]
- Qiu J, Chen S, Chengyou D. A systematic review of robotic-assisted liver resection and meta-analysis of robotic versus laparoscopic hepatectomy for hepatic neoplasms. Surg Endosc 2016;30:862-75. [Crossref] [PubMed]
- Huang JW, Su WL, Wang SN. Alternative Laparoscopic Intracorporeal Pringle Maneuver by Huang's Loop. World J Surg 2018;42:3312-15. [Crossref] [PubMed]
- Heemskerk J, van Gemert WG, Greve JW, et al. Robot-assisted versus conventional laparoscopic Nissen fundoplication: a comparative retrospective study on costs and time consumption. Surg Laparosc Endosc Percutan Tech 2007;17:1-4. [Crossref] [PubMed]
- Fretland ÅA, Dagenborg VJ, Bjørnelv GMW, et al. Laparoscopic Versus Open Resection for Colorectal Liver Metastases: The OSLO-COMET Randomized Controlled Trial. Ann Surg 2018;267:199-207. [Crossref] [PubMed]
- Aghayan DL, Kazaryan AM, Dagenborg VJ, et al. Long-Term Oncologic Outcomes After Laparoscopic Versus Open Resection for Colorectal Liver Metastases: A Randomized Trial. Ann Intern Med 2021;174:175-82. [Crossref] [PubMed]
- Kamarajah SK, Bundred J, Manas D, et al. Robotic versus conventional laparoscopic liver resections: A systematic review and meta-analysis. Scand J Surg 2021;110:290-300. [Crossref] [PubMed]
Cite this article as: Nadkarni S, Patkar S, Goel M. Robotic left hepatectomy for colorectal liver metastases with modified rubber-band technique: surgical technique & steps. J Vis Surg 2023;9:49.