Establishing a robotic coronary artery bypass surgery program: a narrative review
Introduction
Despite not routinely adopted, robotic coronary artery surgery is an established procedure for the management of coronary artery disease (CAD). In the current practice of ageing population, technical improvements of stents deployment and excellent results of guideline directed optimal medical therapy, an increase patient population with moderate CAD could be treated with robotic coronary artery bypass grafting (CABG) alone or in combination with stents (hybrid procedure) to widespread the benefit of the left internal thoracic artery (LITA) to the left anterior descending artery (LAD) rather than receiving multivessels percutaneous coronary intervention (PCI) (1,2). The number of robotic coronary surgery programs around North America has seen a steady growth (3) due to the benefits of the procedure ranging from small incision to fast postoperative recovery.
Traditional cardiac surgical procedures such as CABG have learning curves and prognostic determinants which have been widely studied (4,5). However, learning curves analysis is yet to be established for robotic CABG. In addition, the precise steps to follow in order to build a successful robotic CABG program remain a controversial topic. Another concern is how a stepwise based program for robotic minimally invasive direct coronary artery bypass (MIDCAB) and totally endoscopic coronary artery bypass (TECAB) could provide residents, clinical fellows and surgeons with critical knowledge and clinical practice to become successful robotic coronary surgeons. The main goal of this manuscript is to provide an overview on how to build a successful robotic CABG program and analyze its learning curve. In addition, a literature review was performed to determine the 30-day clinical outcomes of robotic CABG. We present the following article in accordance with the Narrative Review reporting checklist (available at https://jovs.amegroups.com/article/view/10.21037/jovs-22-11/rc).
Robotic CABG: TECAB vs. robotic MIDCAB
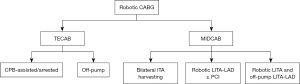
TECAB is the accomplishment of both the take down of the internal thoracic arteries (ITAs) and the completion of the coronary anastomosis with the aim of the robotic platform. Robotic MIDCAB is the combination of the take down of ITAs robotically followed by the deployment of the grafts to the coronary targets via a small thoracotomy. One of the main benefits of TECAB consists of the possibility of serving multiple coronary territories, including the lateral wall and sequential diagonal grafting. With robotic MIDCAB, although a bilateral internal thoracic artery (BITA) case is feasible, the small thoracotomy generally limits the extension of coronary surgery so that most of the robotic MIDCAB ends up receiving LITA to LAD. Therefore, ideally, every robotic surgeon should embrace TECAB to be able to serve more patients with one or two ITAs deployed to the best left coronary artery targets (Figures 1,2).
Survival outcomes robotic MIDCAB vs. TECAB
Methods and research of the study
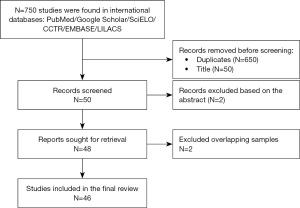
We conduct literature research to compare survival rate after MIDCAB and TECAB. The literature review was done to evidence the clinical outcomes of already established robotic CABG programs. These databases were explored for studies meeting our inclusion criteria and published by January 30th, 2021: PubMed/MEDLINE, Embase, SciELO, LILACS, CCTR/CENTRAL and Google Scholar. We searched for the following terms: [“Robotic CABG” OR “MIDCAB” OR “TECAB” OR “robotic-assisted CABG”]. We identified the studies based following these steps: (I) identification of titles of records through database search; (II) removal of duplicates; (III) screening and selection of abstracts; (IV) assessment for eligibility through full-text papers (Figure 1). Exclusion criteria were: (I) case reports; (II) reviews; (III) articles not in English language; (IV) studies after 2008 when robotic procedures reports began to be published. The study inclusion criteria included: patients with coronary artery disease undergoing robotic CABG with MIDCAB or TECAB.
Results of the study
After excluding duplicates and redundancy, we found 46 studies in a period ranging between 2008–2022 with a total of 9,228 patients (Figure 1). TECAB was performed in 17 studies with a total of 5,321 patients and MIDCAB was performed in 22 studies with a total of 3,066 patients. In addition, we found 5 mixed studies (MIDCAB and TECAB) with a total of 841 patients. Survival rate for MIDCAB ranged between 92–100% for TECAB and between 86–100% for MIDCAB. In addition, mixed studies reported a survival rate oscillating between 75–100% (6-30).
The learning curve for robotic CABG
The high-quality 3D visual feedback from the Da Vinci™ system provides an excellent visual response and indemnification through the visualization of tissue rearrangement and contortion. The administration of robotic technology to CABG also provides a full continuum of advanced technical skills (31-40). This is due to the surgeon skills and to the lack of tremor movement combined with high-powered and magnified vision of the robot. Gain dexterity in using visual clues without perceptive and tactile feedback for the robot-assisted LITA harvest while gaining confidence with carrying out beating-heart off-pump LITA-LAD grafting through a 3–4 cm antero-lateral thoracotomy are crucial steps to technically perform this surgical approach (40). There is a myriad of pathways regarding the learning curve experience for both robotic-assisted MIDCAB and TECAB. Balkhy et al. (41) suggested the following eight steps to successfully become a robotic coronary surgeon.
- Have a good proficiency in off-pump CABG, multi-arterial CABG and skeletonized internal thoracic arteries harvest;
- Proficiency in peripheral cardiopulmonary bypass (CPB) and myocardial protection;
- Robotic training. Skeletonized LITA harvesting during sternotomy cases;
- Single-vessel robotic MIDCAB;
- Cadaver training in robotic TECAB;
- Team simulation in TECAB;
- Single vessel robotic TECAB;
- Multi-vessel robotic TECAB.
At first glance, this list may transmit the idea that TECAB is not a procedure easily mastered by everyone, and surgical experience plays a major role. In addition, some key definitions on what a proficient surgeon is in either off-pump CABG or robotic-assisted MIDCAB, remain unclear (41-48). Although robotic-assisted MIDCAB and TECAB are technically more demanding, advantages such as preserving the sternum, faster recovery, and total graft anastomosis with minimal incisions, have been widely demonstrated (49,50). Another aspect of analyzing a learning curve is how proficient a surgeon is in learning and replicating under supervision each step of the robotic procedures and later performing successfully that procedure independently. Schachner et al. (51) dissected six different moments of the TECAB procedure that need to be learned and replicated and these include:
(I) Lipectomy of the fatty tissue in the anterior mediastinum; (II) pericardiotomy; (III) LITA takedown; (IV) right internal thoracic artery (RITA) takedown; (V) target vessel preparation, with Potts scissors before and after stab incision for arteriotomy; (VI) partial or complete suturing of the LITA to LAD.
According to Schachner et al. (51), after receiving proper training, robotic CABG procedures can be carried out within adequate time limits and yield acceptable results. The authors recommend 100 robotic-assisted MIDCAB and 75 TECAB procedures to be carried out for a surgeon to be able to teach residents and trainees. On the other hand, a retrospective analysis (52) reported a procedural safety after 50 operations for TECAB. In addition, a report from the Society of Thoracic Surgeons (STS) Registry including robotic-assisted MIDCAB procedures performed by 114 surgeons between 2014–2019 revealed that positive clinical outcomes can be attained after the 10th surgical procedure and case sequences >10 were reported to have a reduced rate of surgical approach conversion [odds ratio, 0.27; 95% confidence interval (CI): 0.09–0.84] and upgraded procedural success (odds ratio, 1.96; 95% CI: 1.00–3.84) (53). Overall, the starting of a robotic coronary program should be embraced by a dedicated coronary artery surgeon with deep interest for minimally invasive technique and to expand the portfolio of procedures that she/he is capable to offer for his own patients. As proofed, in several niche of cardiac surgery (aortic surgery, Ross procedure, mitral valve repair), a high-quality center is established among a heart team dedicated to the treatment of coronary artery disease with several tools including guideline directed medical therapy, advanced PCI, minimally invasive robotic CABG and sternotomy multi-arterial off pump CABG.
Selecting patients for robotic-assisted MIDCAB and TECAB
Robotic CABG is a tool in the hands of the heart-team aiming to offer the benefit of LITA to LAD to a widespread of patients. At Lankenau, robotic CABG is not intended to compete with conventional sternotomy CABG, but rather as an ally to better serve patients that would receive multivessels PCI, left main artery stenting or CABG X2 with LITA and one vein, or in patients too fragile to tolerate a conventional sternotomy CABG. In our center, patient candidates for either robotic CABG or coronary intervention are discussed on medical rounds together with the heart-team including cardiac surgeons, interventional cardiologists and anesthesiologists. Based on the risk profile, suitable anatomy, and SYNTAX score, the patient is considered a candidate for either robotic CABG, hybrid revascularization or PCI.
Proximal LAD disease is the main disease treated with robotic CABG, with adjunct coronary disease treatments suitable for stent in hybrid revascularization. For instance, a patient with a chronic total occlusion of the right coronary artery (RCA) not suitable for stenting is not a good candidate for robotic CABG but should be treated through full sternotomy CABG. However, depending on the expertise of the interventional cardiologist hybrid revascularization can also be considered in this population. Robotic CABG offers the possibility to use more BITA in diabetic patients in which the risk of wound infection would be too high.
Finally, despite literature mentioned the need for a number of CT measurements to adopt robotic CABG, in our clinical practice, we actually do not use them but rather have no absolute counterindication. Redo chest, pectum excavatum, frozen left chest from previous left thoracotomy/lung surgery, poor tolerance to single lung ventilation, complete stenting of LAD, intramyocardial LAD, planning for endarterectomy of LAD are relative counterindications that should be carefully reviewed before offering robotic CABG and embraced only by expert team and not in the early stage of the learning curve.
The ideal pathway of selection consists of: (I) patient with stable coronary artery disease; (II) double vessels disease with a non-LAD target that can be treated with stent; (III) robotic CABG LITA to LAD followed by stent of the non-LAD territory with angiographic confirmation of LITA to LAD patency; (IV) adding a second ITA should be evaluated carefully and only after at least 75/100 cases of single LITA to LAD. At Lankenau Heart Institute, robotic-assisted MIDCAB as a stand-alone procedure or as part of hybrid revascularization accounts for 40% of the total annual volume while our surgical approach has been previously described (54). In addition, using a robotic platform enhances the possibility of serving obese patients by allowing a more precise visualization of the proximal LITA during its harvest.
A CT-scan of aortic and aortoiliac vessels is recommended for all patients undergoing peripheral cannulation for CPB with risk factors for peripheral vascular disease (55). When investigations reveal the presence of severe aortic and peripheral vascular disease the operator should avoid peripheral cannulation and retrograde CPB perfusion. Blazek et al. conducted two randomized controlled trials comparing MIDCAB to PCI with bare-metal and drug-eluting stents, respectively. Clinical outcomes from these trials did not evidence differences in the primary composite outcome between revascularization strategies. At 10 and 7 years following the interventional procedure, MIDCAB patients experienced a lower rate of target vessel revascularization than patients treated with PCI bare-metal stent (11% vs. 34%) and drug-eluting stent (1.5% vs. 20%), respectively (56,57).
Indicators of good surgical candidates should be mastered by novel surgeons for them to have the necessary insight to select the right patients for minimally invasive procedures. Patients with a high-risk profile have higher chances to experience unfavourable outcomes, therefore identifying risk factors in patients with low STS score would benefit the surgeon in the decision-making process.
Preliminary steps to build a successful robotic CABG program
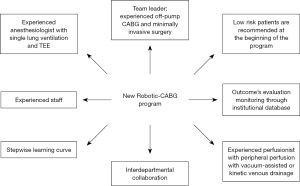
A stepwise approach and a dedicated team are essential for successful procedure implementation (Figure 2). By the time we decided to establish the robotic CABG program at Lankenau, we think that the Donabedian triad approach could provide an enhanced multidisciplinary quality of care. The idea behind the Donabedian’s work underlines the importance of analyzing the healthcare delivery process through a complete assessment of the quality of patient care. It is the allegiance and accuracy with which protocols and procedural interventions are executed, which generates high standard outcomes for patients while welcoming simultaneously patient safety. The goal of this venture was to implement a full-scale patient-centered cardiac care program starting with a focal point on the cardiac robotic CABG. Further implementations included a new policymaking, coordinated and patient-centered program. In addition, a culturally competent system of care was also implemented. Team simulations and training were also conducted at all phases of patient care. A step-by-step process consisting in (I) reaching a high-quality standard level of team training and (II) operating experience was undertaken. After achieving these goals, we began an informative and publicity campaign to broadcast to the medical community and to the public the presence of this robotic program in our center. Robotic CABG creates a unique setting where all the team members including surgeons, anesthesiologists, and nurses face technical and logistic challenges of a new procedure, relying on the team assistance and medical knowledge. Bonatti et al. (58) advised five major steps to be taken including (I) robotic LITA harvesting during CABG through sternotomy; (II) robotic-assisted CABG through minithoracotomy; (III) open chest robotic anastomoses; (IV) TECAB on the arrested heart; (V) totally endoscopic CABG on the beating heart. Seemingly, Rodriguez et al. (59) stressed the importance of a dedicated team and a well-trained cardiac surgeon with solid coronary revascularization experience, which may include off-pump techniques and, ideally, coronary revascularization approaches with minithoracotomy. The surgeon should complete a cardiothoracic surgical training in a program where the surgical management of coronary artery disease is the main focus. We recommend beginning a robotic program by selecting low operative risk-patients with simple pathology. The anesthesiologist must have significant experience with transesophageal imaging, being comfortable with management of single-lung ventilation and having been involved in at least 20 robotic procedures. In addition, the perfusion team should be comfortable with management of peripheral perfusion with vacuum-assisted or kinetic venous drainage. A successful program relies on the surgeon capability to lead the team, providing them with a 360-degree overview of the procedure and explaining technical details. At Lankenau Heart Institute, we adopt the Bluetooth communication technology system during the robotic cases to implement soft communications among all team members. This is essential because in robotics, the surgeon sits away from the patient and, to overcome this limitation, we need to implement perfect communication among team members. Regularly scheduled group meeting should take place to provide the team with visual and comprehensible insights of the procedure, of the medical literature and surgical equipment. Team training is the most important step and pre-planned protocol should be established in advance. A careful selection of team members based on their experience and real interest in the program is highly advisable. Team synergy and attention to details are key to the program success. It is recommended that every team member must have training in the operating room with the robot either on cadavers or animals. This experience would allow a better understanding of the complexity of the procedure. Another important point is the synergistic collaboration among the surgeon, the hospital administration, and the chief of the department, defining reasons for developing a robotic surgical program and setting future goals for the team and the department (Figure 3). These final steps are crucial for the program success and are closely related to the costs of the Da Vinci Xi robot (Intuitive Surgical, Sunnyvale, CA, USA) of 1.5 million US dollars. Barbash et al. (60) estimates that using the robot adds an extra cost of 6–13% for each operation. In addition, Morgan et al. (61) reports that the overall outcomes of the robot may justify the price.
In our experience of more than 2,200 robotic coronary procedures, the cost/benefit of the procedure is achieved thanks to early discharge of these patients (average post operative day 3) and overall availability of the operating room, intensive care unit (ICU) and hospital resource utilizations, including ICU beds, nurse team availability and turnover, that become readily available for other patients. In addition, the lower incidence of postoperative arrhythmias and transfusion requirements further reduces the total hospital costs.
However, the success of a program depends on the presence of certain characteristics such as intellectual capacity, honesty, and commitment.
Promoting the program and the ongoing surgical training
To promote a program, it is vital to follow some important steps such as tracking your own data, create your own database, engage in open discussion with your own interventional cardiologist and internal cardiology department (IC) of the area to make them embrace the concept of hybrid. Once established a solid program there is no need of active campaign because your data and roll results will speak by themselves. It is also important to always take a picture of the LITA to LAD at the time of hybrid procedure. In addition, advertisement is positive for both surgeons and the hospital, history has shown us that with new procedures the term “caution” remains a must until the team has gained sufficient working experience and results have proven to be satisfactory (19). Once the center has reached satisfactory outcomes, we advocate that it organizes cardiac conferences and meetings inviting family doctors and specialists from the region, in order to supply coordinated care and make sure that any patient who has cardiovascular disease is properly screened, diagnosed, and referred appropriately (62). In addition, internet marketing, revamping of hospital and program website, advertisements on television and radio can support further acknowledgment among the community (62). With respect to the surgeon, a continuous update on new technologies, hands-on experience on boot camps, wet labs, participation in international meetings, and proctoring are highly advisable for beginners. In addition, training courses that provide robust interactions between surgeons and experienced leaders remain a must. Surgeons and medical institution should consider monitoring their outcomes through an institutional database, paying special attention to technical gaps, implementing collaboration between team members, and recording videos of the procedures for posterior evaluation. In case the outcomes are not satisfactory, the surgeon should go back to retraining on a robotic clinical fellowship at an experienced center. This training would allow the surgeon to have a deeper understanding of the technical issues and enable the surgeon to carry out the procedures with improved technical skills. Resident training is also fundamental to the development and spreading of robotic programs. In the last years robotic CABG education has changed from being mainly vendor facilitated to a current training condition with minimal direct vendor interaction. After initial robotic cardiac training consensus recommendations, vendor-based training role has diminished, and the application of robotic CABG has evolved. In this context, a better visualization of surgical field and ease to stimulate the surgery have increased the trainee capability to have a 360-degree overview and control of the operation field.
Benefits of robotic-assisted MIDCAB and TECAB
Clinical studies have pointed out the benefits of robotic MIDCAB over non robotic MIDCAB including: (I) a 3–4 cm thoracotomy or ports only; (II) no rib spreading; (III) better visualization of the entire length of ITA; (IV) filtration of surgeon hand tremor; (V) better teaching capabilities due to visualization on a console (54). In addition, non-robotic MIDCABG does not allow to take down a RITA in all comers and to serve every patient independently from the body mass index (BMI): taking down an ITA in an obese patient via a thoracotomy is a very complex procedure. Yanagawa et al. (63) reported a shorter length of stay (5 vs. 6 days; P<0.001), and a lower incidence of mortality (10% vs. 19%; P<0.001) in robotic CABG compared to standard CABG. A propensity-matched analysis of more than 14,000 patients evidenced (21) a higher incidence of in-hospital mortality (21% vs. 11%, P=0.029), acute kidney injury (158% vs. 123%, P=0.0079), transfusion (243% vs. 110%, P=0.0079), postoperative hemorrhage (259% vs. 203%, P=0.044), and length of stay (93±66 vs. 73±62, P<0.01) in the non-robotic CABG group compared to the robotic assisted CABG group. Compared to nonrobotic surgery, robotic-assisted CABG report lower stroke and transfusion rates (64). Kitahara et al. (43) reported similar outcomes among octogenarians and young patients with a higher mortality incidence in the latter. These results confirm the noninferiority of robotic CABG compared to standard CABG.
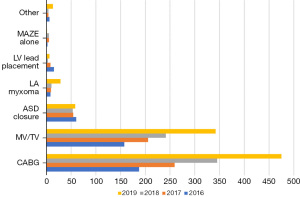
Overall, Europe has seen an increasing trend of the use of cardiac surgery for cardiac surgery (Figures 4,5) (65), while North America had a more stable trend over the years (Figure 6) (49,66). However, the latest North American available data on this trend are a decade old. In this context, the majority of the centers perform 1–5 cases per year while there are only a few centers that perform more than 10 cases per year. The largest clinical study to date on robotic CABG (65,67,68), reported a 2.1% reoperation for bleeding, no perioperative stroke, 0.6% of in-hospital mortality and a 6.4-day length of stay. Most importantly, 98.7% of the surgical procedures were off-pump. Overall, survival rate post robotic CABG is satisfactory.
The forced industrial halt of TECAB
Prometheus, the son of Titan Iapetus, was the Greek champion of human kind that defied the Olympian gods by stealing the fire from them and giving it to humanity in the form of technology, knowledge, and more generally, civilization. In a similar way, the Endowrist™ (Intuitive Surgical, Sunnyvale, CA, USA) stabilizer was vital to TECAB operations. Unfortunately, since the introduction of the new robotic platform Da Vinci Xi™ in 2014, Intuitive has suspended the production of the EndoWrist™ Robotic Stabilizer, therefore greatly limiting TECAB in the current era.
The Endowrist™ stabilizer was originally conceived as part of the instrument set needed to perform robotically TECAB grafting. It allowed the following steps:
- Stabilization of the distal coronary targets during off-pump and on-pump TECAB;
- Harvesting of the proximal portion of the RITA;
- Increase in the antero-posterior diameter of the mediastinum by gently dorsal displacement of the heart to successfully accomplish the robotic harvest of the LITA in those cases with a limited mediastinal space;
- Stabilization of epicardial surface during robotic-assisted myocardial bridge unroofing;
- Lifting the heart during robotic-assisted pericardiectomy for acute relapsing pericarditis;
- Heart positioning during THERESA procedures to ablate left ventricular summit;
- Enhancing exposure during clipping of the left atrial appendage.
In the current practice though the robotic platform Intuitive has withdrawn support to TECAB discontinuing the coronary stabilizer for the Xi Robotic platform system. Without a coronary stabilizer, TECAB cannot be performed. The future is in the hands of a new robotic platform industry that wants to embrace robotic coronary and will offer a robotic stabilizer together with a distal coronary anastomotic device to simplify the procedure.
Two of the largest clinical studies (67,69) reported very few TECAB procedures, likely due the lack of the EndoWrist Stabilizer for the new X and Xi systems which were the most used in Europe. On the other hand, the left-over gap is a huge opportunity for other medical companies that are constantly developing newer designs trying to replace the oldest devices.
Future perspectives
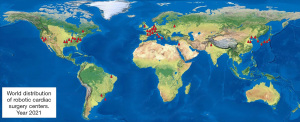
Robotic cardiac surgery has a solid presence in North America, Europe, and Asia but has recently been spreading in Australia, India and South America (Figure 7). A new generation of cardiac surgeons in North America have been pushing the envelope by acting as catalysts for change and integrating robotic platforms in the cardiac surgical training (50). Moreover, a continuous training is mandatory for both trainers and trainees to keep up with technological developments. As such, a cohesive collaboration between team leaders, hospital administrators and industry become crucial to make this endeavour successful.
It is recommended that trainees develop essential instrumental skills including a robust method of instrument position and use. Two clinical studies (70,71) have proven that intensive and console training in performing incision and knot tie reduces the time of the procedure as well as the number of errors. Console instrumental skills need to be developed in a mentored situational environment, first in a drylab and further in a wetlab. Further training advancement can be acquired on the patient bedside as an assistant during the surgical procedure. This includes the port access incision and port position. The next step consists in the acquisition of spatial awareness (understanding the location of organs and structures) as well as the ability to translate the hands movements on the robotic arm movements are crucial. Additional important features include tissues handling, cauterization of the tissues, sawing, and acute bleeding management. It is also important to understand the timing and eventual need of full sternotomy conversion in patient under life-threatening conditions. During the training process in the operating room the trainee should start by performing the simplest part of the procedure and progressively increasing difficult maneuvers as the mentor sees him fit to proceed further.
Robotic CABG is gaining popularity among young surgeons; however, there is a need for call of clinical trials that investigates the current evidence gap for robotic CABG and hybrid revascularization.
Discussion
The long-term results of the SYNTAX trial demonstrated that CABG had fewer major adverse cardiac and cerebrovascular events compared with PCI (69). Initial enthusiasm for robotic CABG has paved the way to new robotic programs in North America and Europe. In our analyses, we described the stepwise pathway to become a robotic coronary surgeon and analyzed the different links between the team and the leader to build a successful program. These include: (I) a curriculum based on previous robotic training and motivated team selected by the leading surgeon; (II) experienced team members; (III) scheduled meetings for team updates; (IV) a successful relationship between hospital administration, chief of the department and team leader; (V) a solid referral basis by a team aimed to support LITA to LAD in the setting of hybrid revascularization or as a stand-alone procedure. The robotic CABG program needs dedicated resources, experts, and stable team members in order to succeed. In addition, it is suggested that only one surgeon performs the operations to maintain high quality standards (59).
The learning curve for surgeons is a stepwise process and surgeons who are confident in performing both on-pump and off-pump CABG are more likely to be successful in this endeavour. LITA harvesting should be the first step in the introduction of this alternative procedure. Further steps include: (I) single-vessel robotic MIDCAB; (II) single vessel robotic TECAB; and (III) multi-vessel robotic TECAB. Training must be proctored, and low risk patients must be selected in the initial phases of the training with a gradual increase over higher risk patients by the end of the training. One of the barriers that are currently affecting the training is the small number of patients in novice surgical programs (59). Therefore, surgical training must be pursued in high-volume surgical programs with expert leaders. At Lankenau Heart Institute, robotic-assisted MIDCAB as a stand-alone procedure or as part of hybrid revascularization accounts for 60% of the total annual volume of CABG (grossly 300+ cases per year), which has remained stable for the past 5 years (54).
In our experience, robotic CABG, in selected patients, was superior to off-pump CABG with sternotomy in the immediate postoperative period and had similar outcomes on the long-term (71). Given our findings from this review, several surgeons and programs may benefit from robotic CABG.
In addition, this study literature review proved once more the benefits of robotic CABG on the survival rate with most of the studies reaching 100% survival (Table 1). However, the recent halt in the production of the EndoWrist Stabilizer poses a severe limitation to TECAB though depriving the patients of a consolidated surgical procedure. However, there is the need for a call for future randomized clinical trials to validate our findings.
Table 1
| Author | Year | Number of patients | Surgical procedure | Survival % |
|---|---|---|---|---|
| Bonatti et al. | 2008 | 5 | TECAB | 100 |
| Holzhey et al. | 2008 | 107/110 | MIDCAB/TECAB | 86 |
| Kiaii et al. | 2008 | 58 | MIDCAB | NR |
| Poston et al. | 2008 | 100 | MIDCAB | NR |
| Reicher et al. | 2008 | 13 | MIDCAB | 86 |
| Kon et al. | 2008 | 15 | MIDCAB | 93 |
| Vassiliades et al. | 2008 | 91 | MIDCAB | NR |
| Gao et al. | 2009 | 6/4 | MIDCAB/TECAB | NR |
| McGinn et al. | 2009 | 450 | MIDCAB | NR |
| Etienne et al. | 2009 | 260 | MIDCAB | NR |
| Sristava et al. | 2010 | 50 | TECAB | NR |
| Bonaros et al. | 2011 | 3/127 | MIDCAB/TECAB | 75 |
| Balkhy | 2011 | 120 | TECAB | 99.2 |
| Jegaden | 2011 | 59 | TECAB | 96.6 |
| Sristava | 2012 | 164 | TECAB | 94.6 |
| Dhawan | 2012 | 106 | TECAB | NR |
| Halkos et al. | 2012 | 269 | MIDCAB | NR |
| Rab et al. | 2012 | 22 | MIDCAB | 95.5 |
| Bachinsky et al. | 2012 | 25 | MIDCAB | 100 |
| Adams et al. | 2013 | 94 | MIDCAB | 88.8 |
| Repossini et al. | 2013 | 166 | MIDCAB | 93.1 |
| Shen et al. | 2013 | 141 | MIDCAB | 93.6 |
| Gasior et al. | 2014 | 98 | MIDCAB | 89.8 |
| Sabashnikov et al. | 2014 | 76 | MIDCAB | 100 |
| Fujita et al. | 2014 | 33 | MIDCAB | NR |
| Daniel et al. | 2014 | 322 | MIDCAB | 99 |
| Halikos et al. | 2014 | 307 | MIDCAB | NR |
| Zaouter et al. | 2015 | 36 | TECAB | 100 |
| Yang et al. | 2015 | 100 | TECAB | NR |
| Choi et al. | 2017 | 80 | MIDCAB | 92.5 |
| Giambruno et al. | 2017 | 203 | MIDCAB | 99 |
| Xia et al. | 2017 | 91 | MIDCAB | 85 |
| Kofler | 2017 | 204 | TECAB/MIDCAB | 100 |
| Balkhy | 2017 | 404 | TECAB | 99.3 |
| Endo | 2019 | 54 | MIDCAB | 100 |
| Balkhy | 2019 | 344 | TECAB | 95 |
| McCrorey | 2019 | 216 | TECAB | 99 |
| Balkhy | 2019 | 28 | TECAB | 100 |
| Kitahara | 2019 | 274 | TECAB | 99 |
| Balkhy | 2020 | 361 | TECAB | 92 |
| Balkhy | 2020 | 440 | TECAB | 99.41 |
| Balkhy | 2021 | 570 | TECAB | 99 |
| Cheng | 2021 | 280 | TECAB/MIDCAB | 96 |
| Balkhy | 2022 | 544 | TECAB | 97.3 |
| Cerny | 2022 | 1266 | TECAB | 99.4 |
MIDCAB, minimally invasive direct coronary artery bypass; TECAB, totally endoscopic coronary artery bypass; NR, not reported.
Limitations
Robotic CABG survival rate has been long debated due to its complexity and the required high skill levels of reproducibility. Therefore, we think that survival outcomes of MIDCAB and TECAB deserve a special mention in this review. On the other hand, only few studies previously reported the outcomes of quality of life and postoperative pain. Therefore, we focused our attention on the survival rate after robotic CABG.
Conclusions
Robotic CABG is a continuously evolving field and new programs are currently in their infancy. Bearing in mind the benefits of the procedure, a stepwise approach is essential for the success of any initial program.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Johan van der Merwe and Filip P. Casselman) for the series “International Perspectives on Minimally Invasive Coronary Artery Revascularization” published in Journal of Visualized Surgery. The article has undergone external peer review.
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jovs.amegroups.com/article/view/10.21037/jovs-22-11/rc
Peer Review File: Available at https://jovs.amegroups.com/article/view/10.21037/jovs-22-11/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.com/article/view/10.21037/jovs-22-11/coif). The series “International Perspectives on Minimally Invasive Coronary Artery Revascularization” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Torregrossa G, Sá MP, Van den Eynde J, et al. Hybrid robotic off-pump versus conventional on-pump and off-pump coronary artery bypass graft surgery in women. J Card Surg 2022;37:895-905. [Crossref] [PubMed]
- Brownlee AR, Amabile A, Torregrossa G, et al. Robotic totally endoscopic triple bypass with bilateral internal mammary arteries and two different anastomotic techniques. J Card Surg 2022;37:249-51. [Crossref] [PubMed]
- Sepehripour AH, Garas G, Athanasiou T, et al. Robotics in cardiac surgery. Ann R Coll Surg Engl 2018;100:22-33. [Crossref] [PubMed]
- Pedersen A, Earley MB, Fenner DJ, et al. How to build a cardiac surgery program. Nurs Manage 2001;32:46-50. [Crossref] [PubMed]
- Lazar HL. Trusting the "Process" to Revitalize a Cardiac Surgery Program. J Am Heart Assoc 2020;9:e019859. [Crossref] [PubMed]
- Bonatti J, Schachner T, Bonaros N, et al. Simultaneous hybrid coronary revascularization using totally endoscopic left internal mammary artery bypass grafting and placement of rapamycin eluting stents in the same interventional session. The COMBINATION pilot study. Cardiology 2008;110:92-5. [Crossref] [PubMed]
- Holzhey DM, Jacobs S, Mochalski M, et al. Minimally invasive hybrid coronary artery revascularization. Ann Thorac Surg 2008;86:1856-60. [Crossref] [PubMed]
- Kiaii B, McClure RS, Stewart P, et al. Simultaneous integrated coronary artery revascularization with long-term angiographic follow-up. J Thorac Cardiovasc Surg 2008;136:702-8. [Crossref] [PubMed]
- Poston RS, Tran R, Collins M, et al. Comparison of economic and patient outcomes with minimally invasive versus traditional off-pump coronary artery bypass grafting techniques. Ann Surg 2008;248:638-46. [PubMed]
- Reicher B, Poston RS, Mehra MR, et al. Simultaneous "hybrid" percutaneous coronary intervention and minimally invasive surgical bypass grafting: feasibility, safety, and clinical outcomes. Am Heart J 2008;155:661-7. [Crossref] [PubMed]
- Kon ZN, Brown EN, Tran R, et al. Simultaneous hybrid coronary revascularization reduces postoperative morbidity compared with results from conventional off-pump coronary artery bypass. J Thorac Cardiovasc Surg 2008;135:367-75. [Crossref] [PubMed]
- Vassiliades TA, Kilgo PD, Douglas JS, et al. Clinical outcomes after hybrid coronary revascularization versus off-pump coronary artery bypass: a prospective evaluation. Innovations (Phila) 2009;4:299-306. [Crossref] [PubMed]
- Gao C, Yang M, Wu Y, et al. Hybrid coronary revascularization by endoscopic robotic coronary artery bypass grafting on beating heart and stent placement. Ann Thorac Surg 2009;87:737-41. [Crossref] [PubMed]
- McGinn JT Jr, Usman S, Lapierre H, et al. Minimally invasive coronary artery bypass grafting: dual-center experience in 450 consecutive patients. Circulation 2009;120:S78-84. [Crossref] [PubMed]
- Etienne PY, Glineur D, Papadatos S, et al. Comparison of minimally invasive direct coronary artery bypass surgery with implantation of drug-eluting stentsin patients with left anterior descending coronary artery disease. Innovations (Phila) 2009;4:340-4. [Crossref] [PubMed]
- Srivastava S, Gadasalli S, Agusala M, et al. Beating heart totally endoscopic coronary artery bypass. Ann Thorac Surg 2010;89:1873-9; discussion 1879-80. [Crossref] [PubMed]
- Bonaros N, Schachner T, Wiedemann D, et al. Closed chest hybrid coronary revascularization for multivessel disease - current concepts and techniques from a two-center experience. Eur J Cardiothorac Surg 2011;40:783-7. [Crossref] [PubMed]
- Balkhy HH, Wann LS, Krienbring D, et al. Integrating coronary anastomotic connectors and robotics toward a totally endoscopic beating heart approach: review of 120 cases. Ann Thorac Surg 2011;92:821-7. [Crossref] [PubMed]
- Jegaden O, Wautot F, Sassard T, et al. Is there an optimal minimally invasive technique for left anterior descending coronary artery bypass? J Cardiothorac Surg 2011;6:37. [Crossref] [PubMed]
- Srivastava S, Barrera R, Quismundo S. One hundred sixty-four consecutive beating heart totally endoscopic coronary artery bypass cases without intraoperative conversion. Ann Thorac Surg 2012;94:1463-8. [Crossref] [PubMed]
- Dhawan R, Roberts JD, Wroblewski K, et al. Multivessel beating heart robotic myocardial revascularization increases morbidity and mortality. J Thorac Cardiovasc Surg 2012;143:1056-61. [Crossref] [PubMed]
- Halkos ME, Walker PF, Vassiliades TA, et al. Clinical and angiographic results after hybrid coronary revascularization. Ann Thorac Surg 2014;97:484-90. [Crossref] [PubMed]
- Rab ST, Douglas JS Jr, Lyons E, et al. Hybrid coronary revascularization for the treatment of left main coronary stenosis: a feasibility study. Catheter Cardiovasc Interv 2012;80:238-44. [Crossref] [PubMed]
- Bachinsky WB, Abdelsalam M, Boga G, et al. Comparative study of same sitting hybrid coronary artery revascularization versus off-pump coronary artery bypass in multivessel coronary artery disease. J Interv Cardiol 2012;25:460-8. [Crossref] [PubMed]
- Adams C, Burns DJ, Chu MW, et al. Single-stage hybrid coronary revascularization with long-term follow-up. Eur J Cardiothorac Surg 2014;45:438-42; discussion 442-3. [Crossref] [PubMed]
- Repossini A, Tespili M, Saino A, et al. Hybrid revascularization in multivessel coronary artery disease. Eur J Cardiothorac Surg 2013;44:288-93; discussion 293-4. [Crossref] [PubMed]
- Shen L, Hu S, Wang H, et al. One-stop hybrid coronary revascularization versus coronary artery bypass grafting and percutaneous coronary intervention for the treatment of multivessel coronary artery disease: 3-year follow-up results from a single institution. J Am Coll Cardiol 2013;61:2525-33. [Crossref] [PubMed]
- Sabashnikov A, Patil NP, Weymann A, et al. Outcomes after different non-sternotomy approaches to left single-vessel revascularization: a comparative study with up to 10-year follow-up. Eur J Cardiothorac Surg 2014;46:e48-55. [Crossref] [PubMed]
- Gąsior M, Zembala MO, Tajstra M, et al. Hybrid revascularization for multivessel coronary artery disease. JACC Cardiovasc Interv 2014;7:1277-83. [Crossref] [PubMed]
- Fujita T, Hata H, Shimahara Y, et al. Initial experience with internal mammary artery harvesting with the da Vinci Surgical System for minimally invasive direct coronary artery bypass. Surg Today 2014;44:2281-6. [Crossref] [PubMed]
- Daniel WT, Puskas JD, Baio KT, et al. Lessons learned from robotic-assisted coronary artery bypass surgery: risk factors for conversion to median sternotomy. Innovations (Phila) 2012;7:323-7. [Crossref] [PubMed]
- Halkos ME, Liberman HA, Devireddy C, et al. Early clinical and angiographic outcomes after robotic-assisted coronary artery bypass surgery. J Thorac Cardiovasc Surg 2014;147:179-85. [Crossref] [PubMed]
- Zaouter C, Imbault J, Labrousse L, et al. Association of Robotic Totally Endoscopic Coronary Artery Bypass Graft Surgery Associated With a Preliminary Cardiac Enhanced Recovery After Surgery Program: A Retrospective Analysis. J Cardiothorac Vasc Anesth 2015;29:1489-97. [Crossref] [PubMed]
- Yang M, Wu Y, Wang G, et al. Robotic Total Arterial Off-Pump Coronary Artery Bypass Grafting: Seven-Year Single-Center Experience and Long-Term Follow-Up of Graft Patency. Ann Thorac Surg 2015;100:1367-73. [Crossref] [PubMed]
- Choi HJ, Kang J, Song H, et al. Comparison of Coronary Artery Bypass Graft-First and Percutaneous Coronary Intervention-First Approaches for 2-Stage Hybrid Coronary Revascularization. Korean J Thorac Cardiovasc Surg 2017;50:247-54. [Crossref] [PubMed]
- Giambruno V, Hafiz A, Fox SA, et al. Is the Future of Coronary Arterial Revascularization a Hybrid Approach?: The Canadian Experience Across Three Centers. Innovations (Phila) 2017;12:82-6. [Crossref] [PubMed]
- Xia Y, Katz AN, Forest SJ, et al. Hybrid Coronary Revascularization has Improved Short-term Outcomes but Worse Mid-term Reintervention Rates Compared to CABG: A Propensity Matched Analysis. Innovations (Phila) 2017;12:174-9. [Crossref] [PubMed]
- Kofler M, Schachner T, Reinstadler SJ, et al. Comparative Analysis of Perioperative and Mid-Term Results of TECAB and MIDCAB for Revascularization of Anterior Wall. Innovations (Phila) 2017;12:207-13. [Crossref] [PubMed]
- Balkhy HH, Nathan S, Arnsdorf SE, et al. Right Internal Mammary Artery Use in 140 Robotic Totally Endoscopic Coronary Bypass Cases: Toward Multiarterial Grafting. Innovations (Phila) 2017;12:9-14. [Crossref] [PubMed]
- Endo Y, Nakamura Y, Kuroda M, et al. The Utility of a 3D Endoscope and Robot-Assisted System for MIDCAB. Ann Thorac Cardiovasc Surg 2019;25:200-4. [Crossref] [PubMed]
- Balkhy HH, Nisivaco S, Kitahara H, et al. Robotic Multivessel Endoscopic Coronary Bypass: Impact of a Beating-Heart Approach With Connectors. Ann Thorac Surg 2019;108:67-73. [Crossref] [PubMed]
- McCrorey M, Kitahara H, Krienbring D, et al. Robotic cardiac surgery impact of a new patient-side assistant on outcomes. Gen Thorac Cardiovasc Surg 2020;68:24-9. [Crossref] [PubMed]
- Kitahara H, McCrorey M, Patel B, et al. Benefit of Robotic Beating-Heart Totally Endoscopic Coronary Artery Bypass in Octogenarians. Innovations (Phila) 2019;14:531-6. [Crossref] [PubMed]
- Balkhy HH, Nisivaco S, Tung A, et al. Does Intolerance of Single-Lung Ventilation Preclude Robotic Off-Pump Totally Endoscopic Coronary Bypass Surgery? Innovations (Phila) 2020;15:456-62. [Crossref] [PubMed]
- Balkhy HH, Nathan S, Torregrossa G, et al. Angiographic patency after robotic beating heart totally endoscopic coronary artery bypass grafting facilitated by automated distal anastomotic connectors. Interact Cardiovasc Thorac Surg 2020;31:467-74. [Crossref] [PubMed]
- Balkhy HH, Nisivaco SM, Hashimoto M, et al. Robotic Total Endoscopic Coronary Bypass in 570 Patients: Impact of Anastomotic Technique in Two Eras. Ann Thorac Surg 2022;114:476-82. [Crossref] [PubMed]
- Cheng N, Zhang H, Yang M, et al. Eleven-year outcomes of U-clips in totally robotic coronary artery bypass grafting versus standard hand-sewn running suture in robotic-assisted coronary artery bypass grafting. Interact Cardiovasc Thorac Surg 2021;33:27-33. [Crossref] [PubMed]
- Balkhy HH, Nisivaco S, Kitahara H, et al. Robotic off-pump totally endoscopic coronary artery bypass in the current era: report of 544 patients. Eur J Cardiothorac Surg 2022;61:439-46. [Crossref] [PubMed]
- Cerny S, Oosterlinck W, Onan B, et al. Robotic Cardiac Surgery in Europe: Status 2020. Front Cardiovasc Med 2022;8:827515. [Crossref] [PubMed]
- Van Praet KM, Kofler M, Shafti TZN, et al. Minimally Invasive Coronary Revascularisation Surgery: A Focused Review of the Available Literature. Interv Cardiol 2021;16:e08. [Crossref] [PubMed]
- Schachner T, Bonaros N, Wiedemann D, et al. Training surgeons to perform robotically assisted totally endoscopic coronary surgery. Ann Thorac Surg 2009;88:523-7. [Crossref] [PubMed]
- Van den Eynde J, Vaesen Bentein H, Decaluwé T, et al. Safe implementation of robotic-assisted minimally invasive direct coronary artery bypass: application of learning curves and cumulative sum analysis. J Thorac Dis 2021;13:4260-70. [Crossref] [PubMed]
- Patrick WL, Iyengar A, Han JJ, et al. The learning curve of robotic coronary arterial bypass surgery: A report from the STS database. J Card Surg 2021;36:4178-86. [Crossref] [PubMed]
- Marin-Cuartas M, Sá MP, Torregrossa G, et al. Minimally invasive coronary artery surgery: Robotic and nonrobotic minimally invasive direct coronary artery bypass techniques. JTCVS Tech 2021;10:170-7. [Crossref] [PubMed]
- Torregrossa G, Amabile A, Balkhy HH. Totally robotic sutured coronary artery bypass grafting: How we do it. JTCVS Tech 2020;3:170-2. [Crossref] [PubMed]
- Blazek S, Rossbach C, Borger MA, et al. Comparison of sirolimus-eluting stenting with minimally invasive bypass surgery for stenosis of the left anterior descending coronary artery: 7-year follow-up of a randomized trial. JACC Cardiovasc Interv 2015;8:30-8. [Crossref] [PubMed]
- Blazek S, Holzhey D, Jungert C, et al. Comparison of bare-metal stenting with minimally invasive bypass surgery for stenosis of the left anterior descending coronary artery: 10-year follow-up of a randomized trial. JACC Cardiovasc Interv 2013;6:20-6. [Crossref] [PubMed]
- Bonatti J, Schachner T, Bonaros N, et al. Robotically assisted totally endoscopic coronary bypass surgery. Circulation 2011;124:236-44. [Crossref] [PubMed]
- Rodriguez E, Nifong LW, Bonatti J, et al. Pathway for surgeons and programs to establish and maintain a successful robot-assisted adult cardiac surgery program. J Thorac Cardiovasc Surg 2016;152:9-13. [Crossref] [PubMed]
- Barbash GI, Glied SA. New technology and health care costs--the case of robot-assisted surgery. N Engl J Med 2010;363:701-4. [Crossref] [PubMed]
- Morgan JA, Thornton BA, Peacock JC, et al. Does robotic technology make minimally invasive cardiac surgery too expensive? A hospital cost analysis of robotic and conventional techniques. J Card Surg 2005;20:246-51. [Crossref] [PubMed]
- Anderson JC, Patel HRH. Robotic Surgery and Successful Set-Up: A Stepwise Approach. Available online: https://www.intechopen.com/chapters/67209
- Yanagawa F, Perez M, Bell T, et al. Critical Outcomes in Nonrobotic vs Robotic-Assisted Cardiac Surgery. JAMA Surg 2015;150:771-7. [Crossref] [PubMed]
- Cavallaro P, Rhee AJ, Chiang Y, et al. In-hospital mortality and morbidity after robotic coronary artery surgery. J Cardiothorac Vasc Anesth 2015;29:27-31. [Crossref] [PubMed]
- Pettinari M, Navarra E, Noirhomme P, et al. The state of robotic cardiac surgery in Europe. Ann Cardiothorac Surg 2017;6:1-8. [Crossref] [PubMed]
- Balkhy HH. Robotic totally endoscopic coronary artery bypass grafting: It's now or never! JTCVS Tech 2021;10:153-7. [Crossref] [PubMed]
- Kilic GS, Walsh TM, Borahay M, et al. Effect of residents' previous laparoscopic surgery experience on initial robotic suturing experience. ISRN Obstet Gynecol 2012;2012:569456. [Crossref] [PubMed]
- Leyvi G, Schechter CB, Sehgal S, et al. Comparison of Index Hospitalization Costs Between Robotic CABG and Conventional CABG: Implications for Hybrid Coronary Revascularization. J Cardiothorac Vasc Anesth 2016;30:12-8. [Crossref] [PubMed]
- Angell J, Gomez MS, Baig MM, et al. Contribution of laparoscopic training to robotic proficiency. J Endourol 2013;27:1027-31. [Crossref] [PubMed]
- Kawashima H, Serruys PW, Hara H, et al. 10-Year All-Cause Mortality Following Percutaneous or Surgical Revascularization in Patients With Heavy Calcification. JACC Cardiovasc Interv 2022;15:193-204. [Crossref] [PubMed]
- Torregrossa G, Sá MP, Van den Eynde J, et al. Robotic hybrid coronary revascularization versus conventional off-pump coronary bypass surgery in women with two-vessel disease. J Card Surg 2022;37:501-11. [Crossref] [PubMed]
Cite this article as: Torregrossa G, Dokollari A, Sá MP, Sicouri S, Ramlawi B, Sutter F. Establishing a robotic coronary artery bypass surgery program: a narrative review. J Vis Surg 2023;9:3.