
First description of extended and tailored fluorescence-guided lymphadenectomy during robotic distal pancreatosplenectomy: case report
Introduction
The use of minimally invasive approaches for pancreatic resections has gained acceptance over the last few years, even for complex procedures. Since the first report of a laparoscopic distal pancreatectomy by Cuschieri in 1994 (1), minimally invasive distal pancreatectomy with or without spleen preservation has become the method of choice for the treatment of benign and malignant tumors of the body and tail of the pancreas, with almost all perioperative outcomes favorable when compared to the open traditional approach (2).
Nevertheless, the laparoscopic approach for complex pancreatic resections has several technical limitations inherent to its own technology, such as two-dimensional vision, poor ergonomics and reduced dexterity, that could possibly compromise safety and oncological results specially during vascular dissections and lymphadenectomies (3).
In this context, the robotic assistance during distal pancreatectomies, first performed by Melvin et al. in 2003 (4), emerged as a technology to overcome laparoscopic limitations. With tridimensional high-definition imaging, improved dexterity with “endowrist” articulation of instruments with seven degrees of motion freedom, better ergonomics and stable positioning of the camera system by the surgeon, the robotic distal pancreatectomy can be performed to very closely mimic the traditional open approach procedure and its principles, even during technically challenging cases (2). As a result, the use of the robotic surgical platform increases the rate of spleen preservation on selected cases, reduces the risk of conversion to open approach and is associated with shorter hospital stay, when compared to the laparoscopic approach (5).
Pancreatic duct adenocarcinoma (PDAC) accounts for more than 90% of all pancreatic malignancies and represents the fourth cause of cancer-related deaths worldwide (6). One of the most aggressive solid malignancies, it is characterized by poor response to medical therapy and less than 8% overall 5-year survival (5). Being surgery the only potentially curative therapy for PDAC, both distant and local recurrence after surgical resection is one of the most important challenges on the treatment of this malignancy (7).
Minimally invasive distal pancreatosplenectomies for the treatment of PDAC of the body and tail of the pancreas have become a well-established approach (7). In order to achieve improved oncologic resection and decrease systemic and local recurrence, several technical alternatives have emerged on the last few years, such as the radical antegrade modular pancreatosplenectomy (RAMPS) (8). This procedure, first developed in 2003 by Strasberg et al. to the traditional open approach and promptly adapted to the laparoscopic and robotic approaches, increases the likelihood of obtaining free circumferential margins, increased rates of R0 resections (microscopically negative margins) and improve lymph node (LN) dissection (8-10).
While it is current accepted that a minimum of 12 LNs should be retrieved during distal pancreatosplenectomies for PDAC, during RAMPS procedure (including open, laparoscopic and robotic approaches) the mean LN harvest is described to be 21 LNs (range, 11–30) (8,11).
With the objective of optimizing tangential margins, better understanding the lymphatic drainage of the pancreas and performing a tailored lymphadenectomy during robotic distal pancreatosplenectomies for the treatment of PDAC, we developed a novel technique: the fluorescence-guided extended lymphadenectomy during PDAC surgical treatment. We present this case in accordance with the CARE reporting checklist (available at https://jovs.amegroups.org/article/view/10.21037/jovs-22-32/rc).
Case presentation
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images/video. A copy of the written consent is available for review by the editorial office of this journal.
In this report, we include the video of a fluorescence-guided tailored lymphadenectomy during a distal pancreatosplenectomy we performed (Video 1).
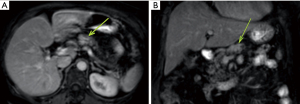
The patient was a 66-year-old female with previous history of high blood pressure, hypothyroidism and grade I obesity [body mass index (BMI) =30.5 kg/m2]. She presented with upper abdominal pain for the last 6 months. Physical examination was normal. She had no previous abdominal surgical procedures nor personal or familial history of digestive cancer or other risk factors relevant to the case (such as tabagism or breast cancer). She was submitted to initial evaluation with an upper abdominal sonography that disclosed a tumor on the pancreatic body. She was further investigated with a magnetic resonance image study of the abdomen that disclosed a 2.5-cm tumor (T2N0M0) staging on the pancreatic body with distal main duct (Wirsung) dilatation (Figure 1). An endoscopic sonography with core needle biopsy was performed and histopathological examination of the samples confirmed a PDAC (Figure 2). Therefore, a distal pancreatosplenectomy was proposed using the da Vinci Xi robotic platform.
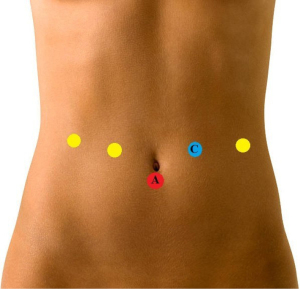
The patient is positioned in supine position with 10 degrees reverse Trendelenburg position with 12 degrees right lateral tilt. Five trocars are routinely used, as disclosed in Figure 3.
The robotic platform is then placed on the left side of the patient.
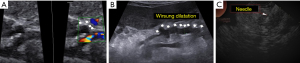
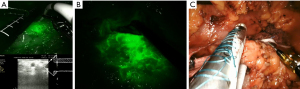
The procedure begins by accessing the retroperitoneal space with the transection of the gastrocolic ligament in order to expose the pancreas. As soon as the pancreatic body is identified, an intraoperative sonography is performed in order to confirm the location of the tumor and its relation to the splenic vessels. Under sonographic guidance, a demarcation on the pancreatic body surface with cautery is made just proximal to the tumor. Then, a 6-Fr ureteral pediatric catheter is inserted on the demarcated spot and 1 mL of indocyanine green (ICG) is injected in the pancreatic parenchyma close to the tumor (Figure 4A). We opted not the make a direct puncture on the tumor (although being oncologically safe, as performed during endoscopic sonography-guided pancreatic tumors biopsies) and inject the ICG close to the tumor to guarantee that the lymphatic drainage of the specific pancreatic area of the tumor would be evaluated. Moreover, the injection site would also be resected, assuring that any pancreatic parenchyma manipulated by the technique would not be spared. Five minutes after the ICG injection, we can evaluate the lymphatic drainage of the pancreatic body with the tumor by using the robotic near infrared real-time fluorescence image mode (FireFly® System) (Figure 4B). Under fluoresce guidance imaging, we could observe clear lymphatic spreading of ICG to the transverse mesocolon, an area not previously suspected to be part of the lymphatic drainage of the pancreas. The area is then demarcated with metallic clips.
The resection begins by usual lymphadenectomy of the stages 8, 9 and 11p LNs according to the 2003 Japanese classification (12). Those LNs also demonstrated intense enhancement under near infrared fluorescence. After that, the pancreatic body is encircled at the level of the splenomesenteric confluence and the pancreas is transected with an endoscopic vascular stapler system with a bioabsorbable stapler line reinforcement tissue (Figure 4C).
The splenic artery is dissected in its origin in the celiac trunk, ligated and transected. Then, the splenic vein is also dissected and transected with an endoscopic vascular stapler. The distal pancreas and the spleen with its vascular pedicles are mobilized from the retroperitoneum and short gastric vessels are transected with harmonic scalpel. After the splenic ligaments are transected, the pancreatosplenectomy is completed.
At this time, we return to the previously demarcated area of the mesocolon that presented intense fluorescence enhancement during the lymphatic drainage evaluation of the pancreatic body and tumor. Using the metallic clips and fluorescence enhancement as references, the area of the transverse mesocolon is resected and the defect created in the mesocolon sutured (Figure 5). We opted to perform the resection of the mesocolon after the pancreatosplenectomy was completed to keep the resection of the pancreas and spleen exactly as we perform in our usual robotic pancreatosplenectomies. Performing an en bloc resection would require some adaptation in the dissection of the inferior pancreatic margin and splenic vein. As this was the first time we performed the fluorescence-guided resection of the mesocolon, we opted to do it after the “regular” procedure was completed successfully.
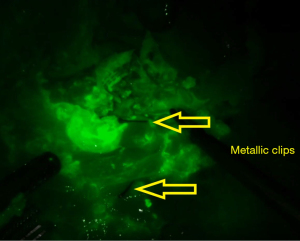
A final evaluation of the area is performed in order to guarantee that al tissues with fluorescence enhancement were resected. The defect created on the transverse mesocolon is then closed with a running suture.
Surgical specimens are retracted inside a plastic bag through a suprapubic incision.
An absorbable hemostatic tissue is placed within the pancreatic and vessels stumps. The procedure is completed with drainage of the area with the pancreatic stump and left subphrenic space.
Discussion
PDACs of the pancreatic body/tail have different characteristics when compared to head tumors. Despite the fact that body/tail tumors are usually larger at diagnosis due to absence of specific symptoms and latter onset of symptoms, they are associated with more aggressive biology and immune avoidance leading to significantly clinical outcomes (13).
Although LN metastasis is an important prognostic factor associated with decreased survival, the role of lymphadenectomy during pancreatectomies is still in intense debate on the international literature. Despite the fact that a few reports concluded that extended lymphadenectomies may provide a survival benefit, it is not clear if the extent of the lymphadenectomy affects survival or oncological results (14). However, a better stablished concept is that extended lymphadenectomies and the number of total LNs retrieval are at least crucial for proper tumor staging and survival prediction (prognostic information) (15).
Another important fact that must be taken into consideration is that most of the investigations focus on lymphadenectomy performed during pancreaticoduodenectomies for the treatment of proximal tumors and it is known that the lymphatic drainage and the routes of LN metastasis are different between the proximal and distal pancreas. In fact, previous studies reported higher incidence of LN metastasis in distal pancreatosplenectomies for the treatment of PDAC along the splenic artery (station 11), celiac trunk (station 9), common hepatic artery (8a/p), para-aortic LNs (station 16), superior mesenteric artery (station 14) and the inferior pancreatic body in up to 35% of the cases (16,17).
The fact that the lymphatic drainage and LN metastasis routes are less studied for distal pancreatectomies, that a previous study reported LN metastasis along inferior pancreatic body (16) and that the extent of the lymphadenectomy may improve oncological results were our motivation to perform an intraoperative evaluation of the pancreatic lymphatic drainage for the treatment of PDAC. Our experience in pancreatic biopsies performed with endoscopic ultrasonography and the technological improvements provided by the robotic surgery platform (such as real time fluorescence and intraoperative sonography with picture-in-picture imaging) were crucial to develop a simple intraoperative method to precisely evaluate the lymphatic drainage of the distal pancreas and tumor. The result was an extended and tailored lymphadenectomy during robotic pancreatosplenectomies for the treatment of distal PDAC.
The most interesting and surprising finding we could observe was the florescence enhancement of the mesocolon (Figure 4B) shortly after the injection of ICG in the pancreatic body. This strongly suggests a not previously suspected lymphatic drainage route of the pancreatic body, not reported elsewhere, and that may explain LNs metastasis along the inferior pancreatic body as reported by Kayahara et al. (16). Moreover, the resection of all fluorescence enhanced tissue, particularly the mesocolon, resulted in a 43 LNs retrieval, while the mean LN retrieval of RAMPS procedure is 21 LNs and the acceptable number of retrieved LNs during pancreatosplenectomies for the treatment of distal PDAC is 12 LNs (8,11).
Our large experience with the use of ICG enhanced real time fluorescence during robotic procedures, intraoperative sonography with picture-in-picture imaging (Figure 4A) and with endoscopic ultrasound-guided pancreatic biopsies may have been critical while performing the technique. It proved to be simple, fast and safe. In fact, bleeding could be considered negligible. The operative time was 4 hours and 43 minutes. The maneuvers performed specifically to the technique we reported lasted for 45 minutes (taking into consideration that this is the first time this technique was performed), including the fluorescence evaluation of the lymphatic drainage of the pancreatic body and tumor (18 minutes), resection of the enhanced mesocolon (14 minutes) and the closure of the defect in the mesocolon (13 minutes). All other retrieved LNs are regularly resected during our robotic distal pancreatosplenectomies (and also presented fluorescence enhancement during our evaluation). All surgical margins were free from neoplasia. Postoperative period was uneventful, and the patient was discharged on the third postoperative day with no pancreatic fistula. The patient did not develop exocrine or endocrine pancreatic dysfunction. Adjuvant chemotherapy was performed and there is no sign of local or distal recurrence 10 months after the procedure on abdominal and chest computed tomography.
This is the first report of a technique for intraoperative lymphatic drainage evaluation of the pancreatic body and tumor, the resection of the mesocolon and tailored lymphadenectomy during pancreatosplenectomies for the treatment of distal PDCA. While clearly disclosing that it results in increased LN retrieval, it is yet to prove if this can result in improved oncological results or better staging of this aggressive type of tumor. We must keep in mind that only the increment on the number of retrieved LNs will not improve oncological results if the correct LNs are not retrieved. However, we resected a tissue that clearly presented fluorescence enhancement containing several LNs, as did the usual LNs resected on distal pancreatosplenectomies (such as stations 9 and 11). Moreover, the resected mesocolon also includes nerves and vessels, and it is yet to evaluate if this can also reduce local recurrence.
Finally, our plan is to perform more cases of the technique we just presented. Further cases may disclose positive LNs on the mesocolon or microvascular and neural invasion. Further studies with proper statistical analysis are necessary to prove if this technique will improve staging and even oncological results.
Conclusions
We describe a new technique of fluorescence guided extended lymphadenectomy with intrapancreatic injection of indocyanine green for the treatment of PDAC of the distal pancreas during robotic distal pancreatosplenectomies. It is a safe method that allows increased LN retrieval and may result in better staging of the tumor and improved oncological outcomes.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jovs.amegroups.org/article/view/10.21037/jovs-22-32/rc
Peer Review File: Available at https://jovs.amegroups.org/article/view/10.21037/jovs-22-32/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.org/article/view/10.21037/jovs-22-32/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images/video. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cuschieri A. Laparoscopic surgery of the pancreas. J R Coll Surg Edinb 1994;39:178-84. [PubMed]
- Velanovich V. Case-control comparison of laparoscopic versus open distal pancreatectomy. J Gastrointest Surg 2006;10:95-8. [Crossref] [PubMed]
- Guerrini GP, Lauretta A, Belluco C, et al. Robotic versus laparoscopic distal pancreatectomy: an up-to-date meta-analysis. BMC Surg 2017;17:105. [Crossref] [PubMed]
- Melvin WS, Needleman BJ, Krause KR, et al. Robotic resection of pancreatic neuroendocrine tumor. J Laparoendosc Adv Surg Tech A 2003;13:33-6. [Crossref] [PubMed]
- Orth M, Metzger P, Gerum S, et al. Pancreatic ductal adenocarcinoma: biological hallmarks, current status, and future perspectives of combined modality treatment approaches. Radiat Oncol 2019;14:141. [Crossref] [PubMed]
- Zhu LL, Wu Z, Li RK, et al. Deciphering the genomic and lncRNA landscapes of aerobic glycolysis identifies potential therapeutic targets in pancreatic cancer. Int J Biol Sci 2021;17:107-18. [Crossref] [PubMed]
- Postlewait LM, Kooby DA. Laparoscopic distal pancreatectomy for adenocarcinoma: safe and reasonable? J Gastrointest Oncol 2015;6:406-17. [PubMed]
- Strasberg SM, Drebin JA, Linehan D. Radical antegrade modular pancreatosplenectomy. Surgery 2003;133:521-7. [Crossref] [PubMed]
- Choi SH, Kang CM, Hwang HK, et al. Robotic anterior RAMPS in well-selected left-sided pancreatic cancer. J Gastrointest Surg 2012;16:868-9. [Crossref] [PubMed]
- Zhou Y, Shi B, Wu L, et al. A systematic review of radical antegrade modular pancreatosplenectomy for adenocarcinoma of the body and tail of the pancreas. HPB (Oxford) 2017;19:10-5. [Crossref] [PubMed]
- Society JP. Classification of pancreatic carcinoma. 2nd English edition. Tokyo: Kanehara & Co. Ltd., 2003.
- Dreyer SB, Jamieson NB, Upstill-Goddard R, et al. Defining the molecular pathology of pancreatic body and tail adenocarcinoma. Br J Surg 2018;105:e183-91. [Crossref] [PubMed]
- Schwarz RE, Smith DD. Extent of lymph node retrieval and pancreatic cancer survival: information from a large US population database. Ann Surg Oncol 2006;13:1189-200. [Crossref] [PubMed]
- Morales-Oyarvide V, Rubinson DA, Dunne RF, et al. Lymph node metastases in resected pancreatic ductal adenocarcinoma: predictors of disease recurrence and survival. Br J Cancer 2017;117:1874-82. [Crossref] [PubMed]
- Erdem S, Bolli M, Müller SA, et al. Role of lymphadenectomy in resectable pancreatic cancer. Langenbecks Arch Surg 2020;405:889-902. [Crossref] [PubMed]
- Kayahara M, Nagakawa T, Futagami F, et al. Lymphatic flow and neural plexus invasion associated with carcinoma of the body and tail of the pancreas. Cancer 1996;78:2485-91. [Crossref] [PubMed]
- Tanaka K, Nakamura T, Asano T, et al. Pancreatic body and tail cancer and favorable metastatic lymph node behavior on the left edge of the aorta. Pancreatology 2020;20:1451-7. [Crossref] [PubMed]
Cite this article as: Surjan RCT, do Prado Silveira S, Figueira ERR, Ardengh JC. First description of extended and tailored fluorescence-guided lymphadenectomy during robotic distal pancreatosplenectomy: case report. J Vis Surg 2023;9:38.


