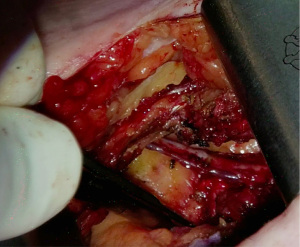
Beating heart multi-vessel minimally invasive direct coronary artery bypass grafting: techniques and pitfalls
Introduction
Coronary artery disease is a prevalent disorder and is one of leading causes of deaths worldwide (1). Coronary artery bypass grafting (CABG) is the gold standard for managing severe coronary artery disease (2). Since its development in 1964 (3), the CABG procedure has been improved to delivery better outcomes and decrease complications for patients (4). One of the improvements is the beating heart Multi-vessel Minimally Invasive Direct Coronary Artery Bypass (Multi-vessel MICS CABG), which has been developed to be a less invasive procedure by obviating the use of a sternotomy. This technique aims to decrease the rates of wound infection, transfusion, post-operative pain, time of recovery and sternal dehiscence, while maintaining the same outcomes as the standard approach with full sternotomy (5,6). Multi-vessel MICS CABG has been a routine approach at our center since 2005 (7). Its experience and outcomes have been reported with excellent results by multiple centers after our initial series (7-11). The aim of this paper is to share the techniques and pitfalls learned during these years of experience.
Patient selection
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the Helsinki Declaration (as revised in 2013). Pictured informed consent was obtained from the patient for publication of this article, the accompanying video and images.
The indication of the revascularization should be decided by a heart team. Whenever feasible and according to the patient’s wish the minimally invasive approach is the option of choice at our center. The selection of a patient for Multi-vessel MICS CABG must be well evaluated pre-operatively. Basically, this selection should take into consideration 3 elements: the surgical exposure, clinical stability during the procedure, and the coronary surgical anatomy. Once there is a good combination of these three elements, we deem that the patient is a candidate for the minimally invasive approach. Otherwise, the patient is selected for a convectional off-pump CABG through a sternotomy.
For the surgical exposure assessment, adequate thorax physical examination should be performed. Chest anomalies, such as severe pectus excavatum, and previous thoracic surgery are usually contraindications for the minimally invasive approach. It is important to inquire about history of pneumonia, chest radiation or trauma which may have led to adhesions and may compromise surgical exposure.
It is mandatory to seek features that may cause clinical instability during the procedure. The patient should be able to keep adequate pulmonary function with single right lung ventilation. Also, heart mobilization to expose the coronary arteries may cause hemodynamic instability in patients with ventricular dysfunction or atrial arrhythmias. Therefore, patients with lung disease associated with decreased pulmonary function tests, patients with significant ventricular dysfunction, and rapid atrial fibrillation are not good candidates for Multi-vessel MICS CABG.
Adequate caliber of the coronary artery target vessels should be present to achieve a good surgical outcome and facilitate the procedure. Ideally the targets should be 1.5 mm or wider. Adequate exposure to the left anterior descending artery (LAD), diagonal artery (Dg), Ramus artery (Rm), obtuse marginal arteries (OM), postero-lateral artery (PL) and Posterior descending artery (PDA) can all be achieved through a small left thoracotomy. The main right coronary artery (RCA), however, is difficult to expose through this approach.
A noncalcified aorta is important in cases where a side aortic clamp is planned to be inserted. The femoral vessels should always be assessed pre-operatively in case femoral cannulation is required for cardiopulmonary bypass (CPB) support.
Operative room preparation
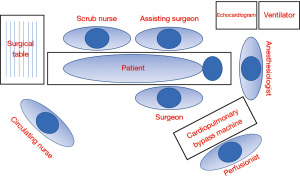
Excellent team synergy is required for this procedure. Good communication and interaction between the main surgeon, surgical assistant, anesthesiologist, perfusionist, and nurses is a must. The procedure is carried on under general anesthesia and monitoring should be done with arterial and central venous line. Intubation strategies, such as double lumen endotracheal tube insertion or bronchial blocker, must be done to allow single right lung ventilation. Perioperative transesophageal echocardiogram is important to monitor cardiac function and to guide cannulation in case that CPB support is necessary.
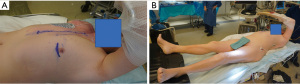
The patient should be positioned in a right lateral decubitus (approximately 25o to 35o). A rolled flannel is placed from the left shoulder to the left hip. The left arm is elevated. The groins should always be easy to access, and also the CPB machine and perfusionist should always be in standby during the procedure in case CPB support is needed. The legs must be exposed for saphenous vein harvest and the right arm when the radial artery is used as a graft. In case the left arm is used, harvest of the radial artery needs to be completed before positioning. Defibrillation external pads should always be placed (Figure 1).
All equipment required for multi-vessel MICS CABG must be available in the Operative room. The check list material used in our institution is presented in the Table 1. Figure 2 shows the layout of the operating room used by our team.
Table 1
| Sternal saw ready for use and a routine coronary surgical instruments box |
| Rultract retractor |
| Knot pusher |
| Octopus handle holder |
| Thoratrak retractor |
| External defib pads |
| Long clip applier |
| Long insulated cautery tip |
| Octopus NUVO |
| Blow mister |
| Starfish |
| Gerald tissues |
| Long coronary scissors |
| Hair clips with holes |
| Long coronary probes |
| Lambert-Kay Aorta Clamp |
Surgical procedure
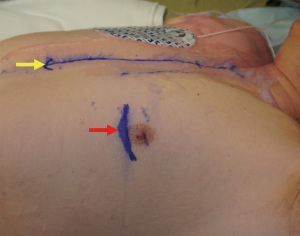
An example of a case can be seen as Video 1. The incision is 4–6 cm long and at the 5th intercostal space on the left midclavicular line (Figure 3). Before starting the procedure, the sternum should be fully exposed in case conversion to sternotomy is required. After reaching the ribs, care must be taken to do not damage the internal mammary vessels while opening the intercostal space. Usually, the 5th intercostal space is the one opened, but if necessary to achieve good surgical exposure either the 4th or 6th intercostal spaces may be opened.
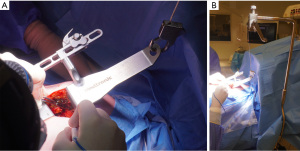
The Thoratrak retractor (Medtronic, Inc., Minneapolis, USA) is placed in the intercostal space. At this point, we may open the pericardium to access the coronary vessels and predict the surgical exposure. After that the assistant usually begins to harvest the venous or radial graft in the usual fashion. The left internal mammary artery (LIMA) is harvested from a lateral approach under direct vision. A special retractor (Rultract Skyhook retractor, Rultract Incorporated, Cleveland, USA) is used to pull the Thoratrak retractor for LIMA harvest (Figure 4). As seen in Figure 4 the Rultract Skyhook retractor is placed in the left side of the patient and the incision is pulled towards the left shoulder. If the LIMA is not easily visualized, dissection of the mediastinal reflection from behind the sternum may help. Skeletonized or pediculated techniques for LIMA harvest may be used according to the surgeon preference (Figure 5). Heparin is administered to achieve an ACT of 280 or more, and next, distal ligation and cut of the LIMA is done.
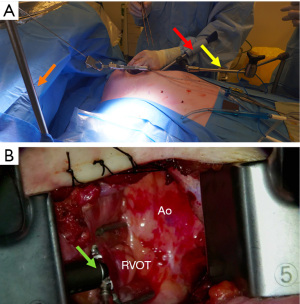
Once the conduits are harvested, exposure of the ascending aorta for proximal anastomoses is initiated. First, the Rultract Skyhook retractor is positioned to the right side of the table to pull the Thoratrak retractor proximally towards the ascending aorta (Figure 6A). Then the pericardium is opened until its superior reflection at the upper part of the ascending aorta. Pericardium traction stitches are placed to bring the aorta closer to the incision and the skin closer to the aorta. A gauze is placed between the aorta and the superior vena cava, which brings the aorta a bit closer to the incision. A 6 mm incision is made inferiorly the main incision in the left 6th or 7th intercostal space. This 6 mm incision allows the introduction of the Octopus NUVO (Medtronic, Inc.), which is positioned over the right ventricular outflow tract (RVOT) to flatten and displace it toward the left posteroinferior direction (Figure 6). Gentle left downward compression on the RVOT generally avoids hemodynamic instability. Once the position is adequate the Octopus is fixed with a holder. Following those steps, the aorta should be accessible to the surgeon and a standard partial occlusion clamp can be placed for proximal anastomoses. Palpation or epicardial scanning prior to clamping should be done to assess the aorta and in cases that aortic disease is suspected clamping should not be performed to avoid the risk of embolic events. In such cases, we perform a composite graft from the LIMA. In cases in which the aorta is not diseased, the side clamping should be placed under a systolic blood pressure of 80–85 mmHg and the proximal anastomosis are performed under direct vision like in the standard fashion using a 6-0 Prolene suture (Figure 7). Often, a knot pusher is used to tie the suture of the proximal anastomosis. Once checked hemostasis of the proximal anastomosis, we take out all the apparatus used for the ascending aorta exposure. At this point, the Rultract Skyhook retractor is no long routinely needed.
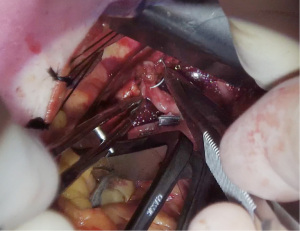
The next step is the distal anastomoses. The blood pressure should be brought up. We aim a systolic blood pressure of 140–150 mmHg. The sequence of distal anastomoses is dictated by the surgeon’s preference and the potential degree of ischemia in each territory.
It is important to mention that for an adequate exposure of the coronary target, the Thoratrak retractor and the Octopus NUVO are used. Complementarily, an armless Starfish Heart Positioner (Medtronic, Inc.) is used whenever the target coronary is the OM, Rm, PL or PDA—please see the Video 1. The Starfish is not needed for the LAD and Dg exposure, as those coronaries are right under the incision and no major heart mobilization is required for that purpose.
For the confection of the distal anastomosis, the Starfish is applied whenever the target vessel is the OM, Rm, PL and PDA through the main incision, and/or the Octopus is applied in the sequence in all target coronaries, using the 6mm incision at the 6th/7th intercostal space (Figure 8). It is crucial to have the holder for stabilization of the Octopus as it permits stability where the distal anastomosis will be performed. In cases in which adequate exposure of the target vessels is not achieved, the use of CPB or conversion to sternotomy should be done. If the patient presents hemodynamic stability with adequate coronary exposure, we procced to the distal anastomosis. For coronary bleeding control, a temporary suture is placed around the coronary artery to be grafted, proximally to the planned arteriotomy. This occludes the coronary for a short period and allows better visualization. A blower is also used to improve visibility of the coronary. After arteriotomy, an intracoronary shunt can be placed and the suture around the coronary may be taken out. It is important not to leave the graft too long by stretching it a bit to the anastomotic site and by cutting with an extra 2 cm or so. Then, the distal anastomosis is performed with a 7-0 or 8-0 Prolene suture with standard instruments. We check all bypass grafts with a doppler flow probe. Protamine is administered after confirmation of adequate graft flow and hemostasis.
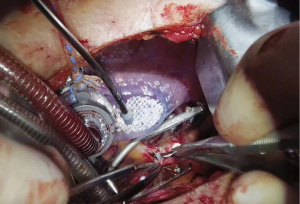
By the end of the procedure, a Blake drain is inserted in the left pleural space via the 6th or 7th intercostal space through the incision where the Octopus was placed. The left lung is reinflated, and proper lie of the grafts and the chest tube should be checked during lung reexpansion. The intercostal space is reapproximated and the subcutaneous tissue and skin are closed. Intercostal nerve blockage is an option to optimize immediate post-operative pain control.
It is important to mention that the priority is (I) safety and (II) efficacy in any surgical procedure. The security of a patient, and the efficiency of a procedure should never be jeopardized due to seeking the “comfort” of a minimally invasive approach. The surgeons performing Multi-vessel MICS CABG should have a low threshold for conversion to sternotomy and/or CPB initiation, especially during their early experience—this is not a failure unless a delayed conversion has already led to a complication. The sternotomy and CPB support should be used in scenarios such as uncontrolled ischemia, bleeding or impossibility of achieve good surgical visualization or exposure.
Clinical outcomes
The outcomes of Multi-vessel MICS CABG have been extensively published by our group (7-9). In our last publication (8), 510 consecutive patients who underwent MICS CABG from September 2005 to December 2020 were analyzed. In this population, the median age of the patients at the time of operation was 64.0 years old and 83% were men. The clinical presentation was acute coronary syndrome in 12% of these patients. In about 82% of these population, the ejection fraction was higher than 45%. Around 16% of the patients required CBP support intraoperatively and in 4% of this cohort conversion to full sternotomy was needed. Median ICU length of stay was 24 h and median duration of hospital stay was 5.0 days. Mortality and major adverse cardiac or cerebrovascular events (MACCE) in the first 30 days after the procedure occurred respectively in 0.2% and in 1.4% of these patients.
Comments
The technique presented in this paper, the beating heart multi-vessel minimally invasive direct coronary artery bypass grafting, is viable, feasible, and a secure option for surgical revascularization in well selected patients with CAD. We strongly believe that Multi-vessel MICS CABG is a reproducible technique that may be applied in multiple expert centers (12).
This paper describes the surgical technique and its steps in details. However, there are pitfalls for all those who perform this procedure. Incorrect rib space for entry is a very common pitfall, that makes surgical exposure inadequate. If needed, the surgeon should not hesitate to open the intercostal space above or below according to his judgment. Another common pitfall is inadequate left lung isolation, which may be solved with bronchoscopy. If not, a wet sponge may be placed on the left lung. When faced by any major complication and adverse scenario, such as uncontrolled bleeding, graft injury, hemodynamic instability, or inability to achieve good surgical exposure remember that conversion to full sternotomy and CPB support are always an option. The minimally invasive approach should never jeopardize the security and safety of the patient.
Further investigation comparing results between the standard approach with sternotomy vs. the minimally invasive approach is needed. No randomized controlled trial has been published yet comparing results between the standard approach with sternotomy vs. the minimally invasive approach. We are leading an international randomized controlled trial called MIST (Minimally Invasive Coronary Surgery Compared to Sternotomy Coronary Artery Bypass Grafting; NCT03447938), which will ascertain whether Multi-vessel MICS CABG provides a faster recovery and equivalent or better long-term outcomes compared with sternotomy CABG (13).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Johan van der Merwe and Filip P. Casselman) for the series “International Perspectives on Minimally Invasive Coronary Artery Revascularization” published in Journal of Visualized Surgery. The article has undergone external peer review.
Peer Review File: Available at https://jovs.amegroups.com/article/view/10.21037/jovs-22-5/prf
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure forms (available at https://jovs.amegroups.com/article/view/10.21037/jovs-22-5/coif). The series “International Perspectives on Minimally Invasive Coronary Artery Revascularization” was commissioned by the editorial office without any funding or sponsorship. MR is a MICS CABG proctor and PI for the MIST trial (both with support from Medtronic, Inc). The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images and videos.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee Circulation 2008;117:e25-146. Erratum in: Circulation 2010;122:e10.
- Paquin A, Poirier P, Beaudoin J, et al. Secondary prevention after CABG: do new agents change the paradigm? Curr Opin Cardiol 2020;35:664-72. [Crossref] [PubMed]
- Kolesov VI, Potashov LV. Surgery of coronary arteries. Eksp Khir Anesteziol 1965;10:3-8. [PubMed]
- Vallely MP, Hameed I, Gaudino M. Commentary: The evolution of coronary artery bypass surgery: Toward a better operation. J Thorac Cardiovasc Surg 2021;162:1122-4. [Crossref] [PubMed]
- Ruel M. Nonsternotomy multivessel coronary artery bypass grafting: A key development in cardiac surgery. JTCVS Tech 2021;10:162-7. [Crossref] [PubMed]
- Ruel M. Commentary: Sternotomy for every cardiac surgery patient ain't the future, so let's get going. J Thorac Cardiovasc Surg 2021;S0022-5223(21)00195-1.
- McGinn JT Jr, Usman S, Lapierre H, et al. Minimally invasive coronary artery bypass grafting: dual-center experience in 450 consecutive patients. Circulation 2009;120:S78-84. [Crossref] [PubMed]
- Guo MH, Vo TX, Horsthuis K, et al. Durability of Minimally Invasive Coronary Artery Bypass Grafting. J Am Coll Cardiol 2021;78:1390-1. [Crossref] [PubMed]
- Lapierre H, Chan V, Sohmer B, et al. Minimally invasive coronary artery bypass grafting via a small thoracotomy versus off-pump: a case-matched study. Eur J Cardiothorac Surg 2011;40:804-10. [Crossref] [PubMed]
- Kikuchi K, Une D, Kurata A, et al. Off-pump minimally invasive coronary artery bypass grafting using the bilateral internal thoracic arteries and the right gastroepiproic artery. Eur J Cardiothorac Surg 2016;49:1285-6. [Crossref] [PubMed]
- Davierwala PM, Verevkin A, Sgouropoulou S, et al. Minimally invasive coronary bypass surgery with bilateral internal thoracic arteries: Early outcomes and angiographic patency. J Thorac Cardiovasc Surg 2021;162:1109-1119.e4. [Crossref] [PubMed]
- Une D, Lapierre H, Sohmer B, et al. Can minimally invasive coronary artery bypass grafting be initiated and practiced safely?: a learning curve analysis. Innovations (Phila) 2013;8:403-9. [Crossref] [PubMed]
- Guo MH, Wells GA, Glineur D, et al. Minimally Invasive coronary surgery compared to STernotomy coronary artery bypass grafting: The MIST trial. Contemp Clin Trials 2019;78:140-5. [Crossref] [PubMed]
Cite this article as: Issa HMN, Ruel M. Beating heart multi-vessel minimally invasive direct coronary artery bypass grafting: techniques and pitfalls. J Vis Surg 2023;9:5.