Anaesthetic considerations for pectus repair surgery
Introduction
Pectus excavatum (PE) and pectus carinatum (PC) is one of the commonest causes of chest wall deformities presenting for surgical correction in thoracic surgery; representing over 95–97% of cases (1). While vast majority are of cases are idiopathic, about 3–4% cases (1) may represent manifestation of congenital syndromes such as Poland syndrome, pentalogy of Cantrell, Spondylothoracic dysplasia (Jarcho-Levin syndrome) or asphyxiating thoracic dystrophy (Jeune syndrome). Connective tissue syndromes (2) such as Marfans and Ehler-Danlos syndrome may also present with pectus deformities. Anaesthetic management of pectus repair surgery is a unique challenge to anaesthetists, as it includes a diverse case mix of relatively young patients with a range of pathologic symptoms. In this review we aim to provide evidence based review of current anaesthetic practice and strategies of analgesia in adult population affected with this disorder.
Clinical presentation
Pectus deformities have a male preponderance (male/female ratio 3:2) and occur with an incidence of 1 in 400 live births (2). Although a detailed review of pathophysiology is beyond the scope of this article, a basic understanding of the pathology is essential for any anaesthetist embarking on anaesthetising for a repair of pectus. PE is caused by a dorsal deviation of sternum and third to seventh costal cartilage and ribs, which result in a funnel shaped deformity; while PC is characterised by a protrusive abnormality of the sternum and ribs caused by abnormal growth of ribs and costal cartilage. This results in progressive compressive/restrictive symptoms due to compression of lung and cardiac structures. While a majority of patients are asymptomatic and require surgery for cosmesis, a small minority of patients have symptoms related to progressively worsening cardio respiratory compression (3,4), scoliosis (2) and pain (1). Some patients may have symptoms related to mitral valve prolapse and regurgitation, although there is not an independent association between both mitral disease and pectus deformities (5). Coln et al. found a 95% incidence of ventricular compression in a case series of 123 patients with pectus (5). Cardiac arrhythmias (6,7) such as second degree heart block, right bundle branch block, Wolff-Parkinson-White syndrome, and exercise induced ventricular arrhythmias (8) have also been described. The severity of symptoms may become progressively worse in patients with a severe pectus deformity (9). Surgical correction in pectus patients aim to reverse these symptoms (10) and enhance body image (11).
Surgical techniques for correction of pectus
Surgery remains the keystone to correction of PE/PC in patients. Decision regarding timing of surgery is made based on a multidisciplinary approach taking into consideration symptoms, signs of cardiac and respiratory compression and patient choice. The severity of the pectus can be measured by the Haller index, which is obtained by dividing the lateral diameter by the antero-posterior diameter. The condition is said to be severe when it is >3.25 (normal value: 2.56) (12). There is no consensus at to what age surgery should be offered. Therefore these patients may present in paediatric as well as adult anaesthetic practice.
The classic technique of repair of PE described in surgical literature is the ‘open’ Ravitch procedure (13) which was first described in 1949. This involves exposure of the anterior thorax region with resection of the costal cartilages affected bilaterally, the performance of a cross sternal osteotomy with the placing of a stabilizer, and the development of muscular flaps (2). This technique involves extensive surgical dissection therefore requires an effective central neuraxial block like thoracic epidural for post-operative analgesia. Donald Nuss introduced a minimally invasive approach to surgical correction (14). This technique involves bilateral mid axillary incisions and insertion of retrosternal metallic bars, which is then fixed and stabilized to correct the defect using a video assisted thoracoscopic technique.
As surgical techniques evolve there is a wide variety of surgical techniques that are utilised for repair of pectus, but they remain modifications of initial procedures described above [e.g., Leonard modification (15) or Robiseck procedure (16)]. Modern techniques (minimally invasive repair of pectus excavatum) now involve using prosthetic material like a bioabsorbable mesh repair (17). These techniques have now allowed anaesthetists to use innovative and less invasive techniques such as chest wall blocks and paravertebral blocks (PVBs) to enhance patient recovery and outcome after surgery. Although a detailed understanding and review of surgical techniques involved in pectus surgery is beyond the scope of this article, a fundamental understanding of operative technique is vital in planning the anaesthetic technique and formulating a robust analgesic strategy in these patients.
Preoperative anaesthetic assessment
Patients presenting for pectus surgery is a diverse spectrum ranging from the otherwise physiologically robust young patients to patients who are affected with complex genetic syndromes. A robust medical and anaesthetic history should be taken in the pre-operative assessment clinic. Baseline cardiorespiratory functional capacity, and aerobic fitness is known to be diminished in patients with PE (18), and it has to be assessed before surgery. Allergies and intolerance are particularly important. In general, patients with history of surgery during childhood are at a risk of latex sensitisation, although it is more frequent in those with associated spina bifida (19). Metal allergies to implants have been described in medical literature (20). A careful history of allergies to nickel, cobalt or other metals, contact dermatitis should be obtained, as 10–15% of population (21) are sensitive to metals. An incidence of 2.8% (35 patients) of Bar Allergy was reported in a case series of 1,215 patients having a Nuss procedure (22) of which three patients had to be re-operated to have their bar removed. Some institutions have instituted pre-operative allergy testing in high-risk patients prior to surgery (23). Patients with prior nickel allergy can have titanium bars to avoid hypersensitivity; Preoperative assessment clinic also provides an ideal opportunity for patient education, discussions related to the anaesthetic approach, risks and benefits of proposed post-operative analgesic regime, and consent for invasive and semi-invasive monitoring such as arterial cannulation or transesophageal echocardiography (TOE).
General investigations
Patient should undergo standard preoperative haematological and biochemical investigations. Chest X rays have been in the past, a main stay of investigation of pectus repair. These should be carefully studied for not only for assessment of severity of pectus deformity/rib abnormality; but also should be carefully scrutinised for evidence of chronic infection (24). Pectus patients have been known to have recurrent mycobacterium avium complex infections (25). CT scan and MRI imaging have long superseded plain chest films and now are the gold standard for investigation. Haller index derived from measuring anteroposterior and side to side diameter of chest gives an index of severity (6). The position of mediastinal structures mainly the heart and great vessels should be carefully noted as these might be displaced or compressed. Compression of right ventricle is a commonly observed abnormality. In severe cases, the heart may become rotationally displaced into the left hemithorax (26). This displacement can cause mechanical compression and obstruction to normal outflow, which may impede normal stroke volume, especially during exercise (27).
Cardiac and respiratory investigations
For a vast majority of patients a standard 12 lead ECG will suffice. Holter monitoring may be considered for patients who have a history of palpitations. A small number of patients will need further investigations such as transthoracic or TOE. RV compression is a common abnormality (27). Mitral valve prolapse with mitral regurgitation is seen in 20% of patients with PE because of mechanical distortion of mitral annulus, and is restored back to normal in 50% after surgery for pectus repair (28).
Routine preoperative investigation with spirometry is not required as this might be frequently be normal. But this is commonly performed as part of the preoperative workup before considering surgery. Spirometry may show a restrictive defect with reduced forced expiratory volume in 1 second (FEV1). A one review of 557 patients presenting for pectus surgery, Goretsky et al. showed that a 26.1% had an forced vital capacity (FVC) 1 29).
Limitation in exercise tolerance and dyspnoea can be associated with pectus deformity. Cardiopulmonary exercise testing (CPET) has been utilised to assess exercise capacity and physiological parameters in patients with PE/PC with conflicting results (30). Considerable difference in methodology has contributed to these conflicting results (27). A decrease in the O2 pulse (mL/beat) and amount of oxygen that could be delivered (VO2 L/min and Ve L/min) may be significantly below predicted values in patients with PE (3,31). Although this may prove to be a useful research tool for physicians to analyse the severity of symptoms of PC/PE, current medical evidence does not indicate a role for using CPET as a routine perioperative risk assessment tool in patients undergoing pectus surgery.
Preoperative preparation
Standard preoperative starvation guidelines (32) should be followed. Usually no premedication (anxiolytics) necessary, but may be used on case by case basis. A single retrospective cohort study published by Ghionzoli et al. showed a statistically significant reduction (P=0.0408) in post-operative opiate requirement in patients who received premedication with 0.02–0.1 mg/kg dose of lorazepam the day before undergoing Nuss surgery (33).
Multidisciplinary team briefing
Preoperative team briefing is increasingly becoming a standard part of operative practice in many countries across the world after introduction of the WHO checklist (34-36). This is a vital aspect of ensuring safety and quality in surgical/anaesthetic practice (37,38). This briefing should be used to confirm the following points prior to induction of anaesthesia:
- Type of surgical procedure and critical steps anticipated;
- Patient positioning;
- Patient specific medical/anaesthetic/surgical issues;
- Anaesthetic protocol (tubes, lines);
- Plan for lung isolation if required;
- Discussion about use of regional anaesthesia;
- Risk of blood loss and confirmation of cross-matched sample being available in theatre;
- Antibiotic prophylaxis;
- Allergies.
This opportunity will help the team to correctly plan and facilitate anaesthesia around the surgical procedure, and provide a high standard of safe and appropriate patient care during and after the procedure (36).
Anaesthetic management
Standard anaesthetic monitoring (39) which includes 3 lead ECG, pulse oximetry should be used. Non-invasive blood pressure monitoring can be safely used for induction in majority of cases. However, invasive arterial monitoring is recommended prior to commencing surgery. Invasive arterial monitoring allows continuous blood pressure measurement during vital steps of surgery where dissection and manipulation of mediastinal structures occur. Initial intravenous access can be a medium size venflon (20 G/22 G), however, a wide bore i.v. cannula (e.g., 14–16 G) should be established under anaesthesia due to the risk of intra operative major hemorrhage. Central venous access is usually not necessary, but can be considered in complex surgical repair, or if patients co morbidities warrants it. This may also be considered if the patient has undergone previous thoracic/cardiac surgery, where dense adhesions increase the likelihood of blood loss.
Perioperative TOE monitoring
In a critical location, even small depressions of the chest can create significant cardiac dysfunction when the right heart and pulmonary outflow tract are compressed to varying degrees by the depressed sternum (5,32,40). The degree of compression of the right ventricle is often difficult to quantify in patients with preoperative transthoracic echocardiography. Perioperative TOE monitoring of the cardiac function in general and right ventricle in particular should be considered in pectus surgery. A comprehensive examination of the heart, including mitral valve and right ventricle dimensions and function is relevant in pectus surgery patients. The quantification the degree of right ventricular obstruction, monitoring for the manipulation of the ventricle during insertion of the bar (41) is useful. This can be best achieved using the mid oesophageal four chamber view and the right ventricle inflow-outflow view by rotating the probe to 45 degrees. There have been a number of case series reporting feasibility of perioperative TOE during pectus surgery (42,43). These have not only helped the anaesthetist to quantify and document the improvement in right ventricular function but also guide the surgical team in real time when performing retrosternal dissection (44). After insertion of the pectus bar, a TOE examination may help to identify pericardial/pleural collections, myocardial injury, or any iatrogenic valvular damage (43). A comprehensive examination of the mitral and tricuspid valve should also be performed before and after the procedure to exclude valvular injuries.
Anaesthetic technique
The technique and conduct of anaesthesia we discuss is relevant to both an open (Ravitch) and minimally invasive repair of pectus excavatum (Nuss) procedures or its modifications. Under this section we would describe the anaesthetic and analgesic techniques widely used in pectus surgery under separate headings of induction and Central and peripheral neuraxial blockade.
Induction and maintenance of anaesthesia
Although any induction agent can be used; induction of anaesthesia is commonly performed using propofol (1.5–3 mg/kg) with an opiate like fentanyl (0.1–0.2 mcg/kg). Co-induction with small dose of midazolam (1–2 mg) given prior to induction can provide effective anxiolysis and has a propofol sparing effect (45,46) along with reduction in postoperative opiate requirement (29).
Any medium to long acting muscle relaxant can be used (e.g., rocuronium, atracurium, cisatracurium) for tracheal intubation. Rocuronium (1–1.5 mg/kg) has a particular advantage of rapid onset, long effect, minimal histamine release and potential rapid reversal with sugammadex (47) in case of failure to intubate.
Such a balanced anaesthetic induction can be maintained with a mixture of air & oxygen with halogenated gas such as sevoflurane or desflurane which have rapid reversal profile. Nitrous oxide should be avoided because the risk of pneumothoraces during the procedure (48) and also because of its effect on post-operative nausea and vomiting (49) which could be particularly harmful due to the presence of the prosthesis and reconstruction. The anaesthesia could be supplemented with a continuous intraoperative epidural infusion or an intravenous target controlled remifentanil infusion (0.1–0.5 mcg/kg/min). Both these techniques provide excellent intra operative analgesia. Remifentanil infusion has the added advantage of reducing stress response to sternotomy (50), rapid titrability, a favorable context sensitive half time (51) allowing for rapid recovery, reducing coughing (52,53) and bucking on extubation.
Total intravenous anaesthesia (TIVA) using target-controlled infusion of propofol and remifentanil is also a well-established anaesthetic technique. There are no studies comparing balanced anaesthesia with volatile agents and TIVA in pectus surgery. There is some evidence in thoracotomy patients to indicate a potential benefit of using propofol infusion to reduce post thoracotomy chronic pain (53,54). However, this has not been confirmed in large multicentre randomized control trial, therefore still remains a subject of scientific research. It is recommended that depth anaesthesia monitoring (e.g., bi-spectral index or entropy) is used to reduce the risk of awareness (54). If TIVA is used to provide anaesthesia, it is important to administer adequate muscle relaxant irrespective of the technique used to achieve paralysis of the diaphragm, as TIVA does not contribute to diaphragmatic muscle relaxation (55-57).
The choice of tube used for tracheal intubation is dictated by the surgical procedure. Nuss repair requires lung isolation so that the surgeon can dissect the retrosternal space, and correctly position the retrosternal prosthesis with a video thoracoscopic assisted technique. Ravitch procedure on the other hand is an open procedure and a normal endotracheal tube will suffice. However with increased surgical expertise, minimally invasive Nuss procedure can be performed with a normal single lumen endotracheal tube with creation of an artificial pneumothorax with carbon dioxide insufflation (2). If lung isolation is required then a bronchial blocker may be used in these circumstances.
If the surgical correction requires lung isolation, a left sided double lumen tube (2) can be used. Tube position should be confirmed on intubation and once again after positioning the patient with a fiber-optic bronchoscope. Lung isolation might be difficult in patients with Marfans syndrome using a standard tube size and may require a bigger tube size.
Central and peripheral neuraxial blockade
Since the very beginning, central neuraxial blockade was used to provide analgesia for patients undergoing Ravitch procedure because of the potential for severe pain (13,58-61). Although Nuss repair is a minimally invasive thoracoscopic technique, studies cited in medical literature have shown that the perioperative pain is at the least comparable with open repair techniques such as Ravitch procedure (48,59). Pain management following surgery has been shown to affect all measurable objective outcomes during hospitalization, which includes the capacity for deep breathing, the capacity of early mobilization, the possibility to perambulate, and the length of hospital stay (62). Severity of pectus deformity has been shown to be an independent predictor of post-operative pain in patients undergoing surgery (63). Nagasao et al. have demonstrated that pain after pectus surgery has a different distribution in children and adults (64). However, there was no difference in the pain scores of adults and children after surgery.
Thoracic epidural anaesthesia
The standard of perioperative analgesia in most institutions remains a thoracic epidural analgesia (TEA) with a catheter inserted at level T3–5 before anaesthetic induction (2,15,22,65). Epidural analgesia is provided for at least 3 days (66). Thoracic epidural has been found consistently superior compared to intravenous analgesia with opiates in clinical trials resulting in lower postoperative pain scores in conjunction with greater patients well-being (67-70). One study reported a longer operating room time, increase in calls for an anesthetic review, and greater hospital charges with epidural analgesia after repair of PE, but consistently showed a good pain control with TEA (69). However, thoracic epidural can be difficult to insert especially in adults, and is associated with a failure rate of about 30% (71). TEA is also associated with potentially severe complication of epidural hematoma. The exact incidence of epidural hematoma associated with thoracic epidural insertion is difficult to quantify as the important studies estimating the risks of complication after epidural insertion such as NAP3 (72) include lumbar epidurals and a varied case mix patients. A pessimistic estimate in this study suggests a rate of 4.2 (95% confidence interval, 2.9–6.1) per 100,000 patient population.
Epidural opiates such as fentanyl (73), sufentanil (74), diamorphine, morphine (75) are commonly added to improve the quality of analgesia with epidurals. Other adjuncts such as clonidine have been studied in paediatric anaesthetic practice and have found to have a comparable efficacy for patient undergoing Nuss repair (76). This strategy is not without potential complications such as respiratory depression with hydrophilic opiates such as morphine, pruritus, post-operative nausea and vomiting or urinary retention.
Paravertebral block (PVB)
Paravertebral nerve blocks have been commonly performed in thoracic surgery as an adjunct to anaesthesia and analgesia after surgery. Thoracic PVB may be a suitable alternative in thoracotomy patients, where only a unilateral sensory block is desirable (77). Bilaterally inserted PVB (78,79) have been used in paediatric surgical practice. In this study bilateral paravertebral catheters resulted in equivalent opioid consumption and pain scores when compared to TEA for postoperative pain management in pediatric patients undergoing the Nuss procedure. However, larger studies in adult population are lacking. Paravertebral catheters inserted during surgery can be potentially utilised in adult patients as an alternative to epidural catheters in case of an absolute contraindication, or failure to site an epidural. Larger volumes of local anaesthetic (>20 mL) are required for an effective PVB. Paravertebral space being a potentially vascular space, there is a potential risk of local anaesthetic toxicity. Addition of epinephrine to the local anaesthetic solution may delay absorption of local anaesthetic from the space and mitigate the risk of local anaesthetic toxicity. Bilateral PVBs have been successfully used in thoracic surgery and may be an alternative for patients in whom epidural is contraindicated or unsuccessful, but there is a concern about local anaesthetic toxicity due to the large volume of it required for the blocks to be effective (80). Recent studies have suggested a beneficial effect of PVB in reducing the incidence of chronic pain after thoracic surgery and this might be an avenue to explore in pectus surgery (81).
Intercostal nerve blocks
As experience with minimally invasive pectus repair techniques grows, centres across the world have started increasingly using intercostal nerve blocks in combination with multimodal analgesia (NSAIDS + opiates) to treat patients instead of inserting epidural catheters in paediatric population. These studies have reported comparative analgesic efficacy with reduced length of stay and cost benefit (82,83). Again larger randomised controlled studies in adult patients are lacking. Nonetheless, there is evidence of reduced post-operative opiate requirement after intercostal blocks in paediatric population (84). Despite this bilateral intercostal blocks have been used in combination with multimodal analgesic techniques in adults (85,86) in pectus surgery.
Other chest wall blocks
Sternal nerve supply is from the 2nd to 6th intercostal nerve. A number of nerve blocks have been described to provide analgesia after sternotomy. One approach it to perform bilateral parasternal block (87), which is essentially infiltration of local anaesthetic under direct vision. A novel technique of ‘pectoralis block’ (commonly known as ‘PECS Block’), which involves infiltration of local anaesthetic in the fascial plane between pectoralis major and minor has been described by Blanco (88). This has been shown to provide effective analgesia of the thoracic wall comparable to thoracic PVB (89,90). Pecs I block is an interfascial plane block where local anaesthetic is deposited into the plane between the pectoralis major muscle and the pectoralis minor muscle (Pecs I block). If the anaesthetic is also deposit above the serratus anterior muscle at the third rib the technique is described as Pecs II block. These novel techniques attempt to block the pectoral; intercostobrachial; intercostals III, IV, V, VI; and long thoracic nerves. These techniques have been found effective in management of pain after breast surgery (91), and provide exciting alternative to be explored in pectus surgery especially minimally invasive techniques. The “Serratus nerve block” has been recently described in medical literature. This involves an ultrasound guided injection of local anaesthetic superficial or deep underneath serratus anterior. In a volunteer study (n=4) demonstrated dermatomal paraesthesia from T2 to T9 and numbness in all volunteers (92). These new blocks have the potential of being important adjuncts in chest wall surgery and promise to be a good alternative for patients with contraindications for neuraxial techniques such as epidural or PVB. Further research is needed to scope its use in routine clinical practice.
Intraoperative management
Patient should be positioned according to surgical preference. Care should be taken to avoid brachial plexus injuries in patients undergoing Nuss repair (93). In a recent publication of a single centre experience of 90 paediatric patients undergoing Nuss repair reported a 5.2% incidence of brachial plexus injury directly related to positioning (94). Ulnar nerve damage has also been reported in literature (95). Temperature monitoring and external body warmer should be instituted to avoid inadvertent perioperative hypothermia. Fluid management should be goal directed avoiding fluid overload using a balanced crystalloid. Cardiac output monitoring devices such as oesophageal Doppler or direct visualisation of left ventricular volume status with a TOE probe may be utilised for this purpose. All patients should receive antiemetic prophylaxis. A multimodal analgesia approach is paramount and it may include that include paracetamol, non-steroidal anti-inflammatory agent (e.g., ketorolac, Parexocib, etc.) and opiates. The use IV ketorolac given intraoperatively has demonstrated to reduce post-operative opiate requirement in pectus surgery (64).
Perioperative TOE monitoring
In a critical location, even small depressions of the chest can create significant cardiac dysfunction when the right heart and pulmonary outflow tract are compressed to varying degrees by the depressed sternum (5,30,40). The degree of compression of the right ventricle is often difficult to quantify in patients with preoperative transthoracic echocardiography. Perioperative TOE monitoring of the cardiac function in general and right ventricle in particular should be considered in pectus surgery. A comprehensive examination of the heart, including mitral valve and right ventricle dimensions and function is relevant in pectus surgery patients. The quantification the degree of right ventricular obstruction, monitoring for the manipulation of the ventricle during insertion of the bar (41) is useful. This can be best achieved using the mid oesophageal four chamber view and the right ventricle inflow-outflow view by rotating the probe to 45 degrees. There have been a number of case series reporting feasibility of perioperative TOE during pectus surgery (42,44). These have not only helped the anaesthetist to quantify and document the improvement in right ventricular function but also guide the surgical team in real time when performing retrosternal dissection (44). After insertion of the pectus bar, a TOE examination may help to identify pericardial/pleural collections, myocardial injury, or any iatrogenic valvular damage (43). A comprehensive examination of the mitral and tricuspid valve should also be performed before and after the procedure to exclude valvular injuries.
Intraoperative complications in pectus surgery
Although minimally invasive, Nuss repair and its modifications are associated with some important potential complications. Anaesthetist should remain vigilant during and after surgery to diagnose these early and initiate immediate treatments (see Table 1). The major intraoperative complications include perforation of the right ventricle and vascular injuries leading to life-threatening haemorrhage. Cardiac perforation, liver perforation, aortic injury, inferior vena cava compression, intercostal artery bleeding have all been described in literature (22,43,96-100). These can be proactively monitored with intraoperative TOE. Transgastric short axis view and its modifications, and the mid-oesophageal four chamber views of the left ventricle can be best utilised for detection of a cardiac tamponade. Rotation of the TOE probe in the mid oesophageal position can also help in demonstrating presence of fluid in the pleural space, which can alert the surgeons to potential sources of bleeding.
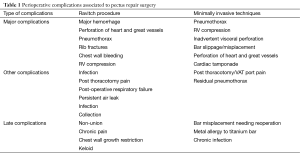
Full table
Probe inserted in deep transgastric position can also help to demonstrate presence of free fluid around the liver capsule and inferior vena cava compression by rotating the probe position to the right. Management of cardiac injuries may require institution of emergency cardiopulmonary bypass (101). Minor complications include lung trauma, pericardial tears, pleural and pericardial effusion that do not require treatment (77-79).
Residual pneumothorax requiring chest drain after the operation is quite common (>50% cases) (77), and some experts advocate deep extubation to avoid coughing in these patients (67).
Postoperative care
If a regional analgesic technique is used (e.g., epidural,) it should be established prior to extubation. Intercostal blocks should be considered for additional drain sites. If epidural is not used then intravenous opiates like fentanyl, morphine (with a patient controlled analgesia system) need to be used as part of multimodal analgesic regimen (53,55,102). Ketamine has been used as an adjunct to IV opiates (85). Intercostal blocks can also be used as a rescue analgesic technique in recovery in cases of epidural failure (103).
Studies have shown that patients with after Nuss surgery still experience significant pain up to day 7 (104) and epidural may need to be supplemented with IV opiates and other adjuncts. A significant proportion of pain after thoracic surgery is neuropathic in origin (105). Gabapentin is a useful adjunct which has been successfully evaluated as an analgesic in thoracic surgery (106-109).
Lung atelectasis and pneumonia in post-operative period is common after repair of pectus (110). High flow humidified oxygen delivered with special nasal cannula (e.g., OptiflowTM) in combination with respiratory care can help in reducing post-operative respiratory complications in this patient group (111-113).
Patients should be encouraged to ambulate early, and undertake regular breathing exercises.
Patients are usually counselled in the preoperative clinics and should be reminded to avoid lifting heavy weights or lying on one side after the procedure. They should also avoid bending at the waist or pushing through their arms (114).
Conclusions
Pectus surgery is carried out across a range of age group. Minimally invasive surgical techniques have increasingly been used to repair the deformities. With increasing surgical expertise, the need for anaesthesia requiring lung isolation has been reduced. Intraoperative monitoring with TOE can minimise the catastrophic complications associated with the procedure. A general anaesthesia supplemented with good analgesic technique is crucial to the success of the surgery and reducing perioperative complications. Analgesic techniques are evolving with many centres now using multimodal techniques, which include regional blocks, newer peripheral nerve blocks, and effective analgesics.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Brochhausen C, Turial S, Müller FK, et al. Pectus excavatum: history, hypotheses and treatment options. Interact Cardiovasc Thorac Surg 2012;14:801-6. [Crossref] [PubMed]
- Molins L, Fibla JJ, Perez J, et al. Chest wall surgery: Nuss technique for repair of pectus excavatum in adults. Multimed Man Cardiothorac Surg 2007;2007:mmcts.2004.000315.
- Colombani PM. Preoperative assessment of chest wall deformities. Semin Thorac Cardiovasc Surg 2009;21:58-63. [Crossref] [PubMed]
- Guntheroth WG, Spiers PS. Cardiac function before and after surgery for pectus excavatum. Am J Cardiol 2007;99:1762-4. [PubMed]
- Coln E, Carrasco J, Coln D. Demonstrating relief of cardiac compression with the Nuss minimally invasive repair for pectus excavatum. J Pediatr Surg 2006;41:683-6; discussion 683-6. [Crossref] [PubMed]
- Haller JA Jr, Loughlin GM. Cardiorespiratory function is significantly improved following corrective surgery for severe pectus excavatum. Proposed treatment guidelines. J Cardiovasc Surg (Torino) 2000;41:125-30. [PubMed]
- Kelly RE Jr. Pectus excavatum: historical background, clinical picture, preoperative evaluation and criteria for operation. Semin Pediatr Surg 2008;17:181-93. [Crossref] [PubMed]
- Chan Wah Hak YS, Lim YP, Liew R, et al. Pectus excavatum: uncommon electrical abnormalities caused by extrinsic right ventricular compression. J Cardiovasc Electrophysiol 2014;25:324-7. [Crossref] [PubMed]
- Fonkalsrud EW, Dunn JC, Atkinson JB. Repair of pectus excavatum deformities: 30 years of experience with 375 patients. Ann Surg 2000;231:443-8. [Crossref] [PubMed]
- Johnson JN, Hartman TK, Pianosi PT, et al. Cardiorespiratory function after operation for pectus excavatum. J Pediatr 2008;153:359-64. [Crossref] [PubMed]
- Kelly RE Jr, Cash TF, Shamberger RC, et al. Surgical repair of pectus excavatum markedly improves body image and perceived ability for physical activity: multicenter study. Pediatrics 2008;122:1218-22. [Crossref] [PubMed]
- Haller JA Jr, Shermeta DW, Tepas JJ, et al. Correction of pectus excavatum without prostheses or splints: objective measurement of severity and management of asymmetrical deformities. Ann Thorac Surg 1978;26:73-9. [Crossref] [PubMed]
- Ravitch MM. The Operative Treatment of Pectus Excavatum. Ann Surg 1949;129:429-44. [Crossref] [PubMed]
- Nuss D, Kelly RE Jr, Croitoru DP, et al. A 10-year review of a minimally invasive technique for the correction of pectus excavatum. J Pediatr Surg 1998;33:545-52. [Crossref] [PubMed]
- Antonoff MB, Erickson AE, Hess DJ, et al. When patients choose: comparison of Nuss, Ravitch, and Leonard procedures for primary repair of pectus excavatum. J Pediatr Surg 2009;44:1113-8; discussion 118-9. [Crossref] [PubMed]
- Robicsek F, Watts LT, Fokin AA. Surgical repair of pectus excavatum and carinatum. Semin Thorac Cardiovasc Surg 2009;21:64-75. [Crossref] [PubMed]
- Luzzi L, Voltolini L, Zacharias J, et al. Ten year experience of bioabsorbable mesh support in pectus excavatum repair. Br J Plast Surg 2004;57:733-40. [Crossref] [PubMed]
- Rowland T, Moriarty K, Banever G. Effect of pectus excavatum deformity on cardiorespiratory fitness in adolescent boys. Arch Pediatr Adolesc Med 2005;159:1069-73. [Crossref] [PubMed]
- Soultanis KC, Payatakes AH, Chouliaras VT, et al. Rare causes of scoliosis and spine deformity: experience and particular features. Scoliosis 2007;2:15. [Crossref] [PubMed]
- Harloff T, Hönle W, Holzwarth U, et al. Titanium allergy or not? "Impurity" of titanium implant materials. Health 2010;2:306-10. [Crossref]
- Thyssen JP, Linneberg A, Menné T, et al. The epidemiology of contact allergy in the general population--prevalence and main findings. Contact Dermatitis 2007;57:287-99. [Crossref] [PubMed]
- Kelly RE, Goretsky MJ, Obermeyer R, et al. Twenty-one years of experience with minimally invasive repair of pectus excavatum by the Nuss procedure in 1215 patients. Ann Surg 2010;252:1072-81. [Crossref] [PubMed]
- Aneja S, Taylor JS, Soldes O, et al. Dermatitis in patients undergoing the Nuss procedure for correction of pectus excavatum. Contact Dermatitis 2011;65:317-21. [Crossref] [PubMed]
- Iseman MD, Buschman DL, Ackerson LM. Pectus excavatum and scoliosis. Thoracic anomalies associated with pulmonary disease caused by Mycobacterium avium complex. Am Rev Respir Dis 1991;144:914-6. [Crossref] [PubMed]
- Kim RD, Greenberg DE, Ehrmantraut ME, et al. Pulmonary nontuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med 2008;178:1066-74. [Crossref] [PubMed]
- Jaroszewski DE, Fonkalsrud EW. Repair of pectus chest deformities in 320 adult patients: 21 year experience. Ann Thorac Surg 2007;84:429-33. [Crossref] [PubMed]
- Fonkalsrud EW, Bustorff-Silva J. Repair of pectus excavatum and carinatum in adults. Am J Surg 1999;177:121-4. [Crossref] [PubMed]
- Shamberger RC, Welch KJ, Sanders SP. Mitral valve prolapse associated with pectus excavatum. J Pediatr 1987;111:404-7. [Crossref] [PubMed]
- Goretsky MJ, Kelly RE Jr, Croitoru D, et al. Chest wall anomalies: pectus excavatum and pectus carinatum. Adolesc Med Clin 2004;15:455-71. [Crossref] [PubMed]
- Jaroszewski D, Steidley E, Galindo A, et al. Treating heart failure and dyspnea in a 78-year-old man with surgical correction of pectus excavatum. Ann Thorac Surg 2009;88:1008-10. [Crossref] [PubMed]
- Malek MH, Fonkalsrud EW, Cooper CB. Ventilatory and cardiovascular responses to exercise in patients with pectus excavatum. Chest 2003;124:870-82. [Crossref] [PubMed]
- Søreide E, Eriksson LI, Hirlekar G, et al. Pre-operative fasting guidelines: an update. Acta Anaesthesiol Scand 2005;49:1041-7. [Crossref] [PubMed]
- Ghionzoli M, Brandigi E, Messineo A, et al. Pain and anxiety management in minimally invasive repair of pectus excavatum. Korean J Pain 2012;25:267-71. [Crossref] [PubMed]
- Haynes AB, Weiser TG, Berry WR, et al. A surgical safety checklist to reduce morbidity and mortality in a global population. N Engl J Med 2009;360:491-9. [Crossref] [PubMed]
- Vats A, Vincent CA, Nagpal K, et al. Practical challenges of introducing WHO surgical checklist: UK pilot experience. BMJ 2010;340:b5433. [Crossref] [PubMed]
- World Alliance for Patient Safety. WHO surgical safety checklist and implementation manual. Available online: http://www.who.int/patientsafety/safesurgery/ss_checklist
- Mahajan RP. The WHO surgical checklist. Best Pract Res Clin Anaesthesiol 2011;25:161-8. [Crossref] [PubMed]
- Schlack WS, Boermeester MA. Patient safety during anaesthesia: incorporation of the WHO safe surgery guidelines into clinical practice. Curr Opin Anaesthesiol 2010;23:754-8. [Crossref] [PubMed]
- Winter A, Spence AA. An international consensus on monitoring? Br J Anaesth. 1990;64:263-6. [Crossref] [PubMed]
- Mocchegiani R, Badano L, Lestuzzi C, et al. Relation of right ventricular morphology and function in pectus excavatum to the severity of the chest wall deformity. Am J Cardiol 1995;76:941-6. [Crossref] [PubMed]
- Becmeur F, Ferreira CG, Haecker FM, et al. Pectus excavatum repair according to Nuss: is it safe to place a retrosternal bar by a transpleural approach, under thoracoscopic vision? J Laparoendosc Adv Surg Tech A 2011;21:757-61. [Crossref] [PubMed]
- Park SY, Park TH, Kim JH, et al. A case of right ventricular dysfunction caused by pectus excavatum. J Cardiovasc Ultrasound 2010;18:62-5. [Crossref] [PubMed]
- Bouchard S, Hong AR, Gilchrist BF, et al. Catastrophic cardiac injuries encountered during the minimally invasive repair of pectus excavatum. Semin Pediatr Surg 2009;18:66-72. [Crossref] [PubMed]
- Krueger T, Chassot PG, Christodoulou M, et al. Cardiac function assessed by transesophageal echocardiography during pectus excavatum repair. Ann Thorac Surg 2010;89:240-3. [Crossref] [PubMed]
- Whitwam JG. Co-induction of anaesthesia: day-case surgery. Eur J Anaesthesiol Suppl 1995;12:25-34. [PubMed]
- Tighe KE, Warner JA. The effect of co-induction with midazolam upon recovery from propofol infusion anaesthesia. Anaesthesia 1997;52:1000-4. [Crossref] [PubMed]
- Lee C, Jahr JS, Candiotti KA, et al. Reversal of profound neuromuscular block by sugammadex administered three minutes after rocuronium: a comparison with spontaneous recovery from succinylcholine. Anesthesiology 2009;110:1020-5. [Crossref] [PubMed]
- Eger EI 2nd, Saidman LJ. Hazards of nitrous oxide anesthesia in bowel obstruction and pneumothorax. Anesthesiology 1965;26:61-6. [Crossref] [PubMed]
- Fernández-Guisasola J, Gómez-Arnau JI, Cabrera Y, et al. Association between nitrous oxide and the incidence of postoperative nausea and vomiting in adults: a systematic review and meta-analysis. Anaesthesia 2010;65:379-87. [Crossref] [PubMed]
- Steinlechner B, Dworschak M, Birkenberg B, et al. Low-dose remifentanil to suppress haemodynamic responses to noxious stimuli in cardiac surgery: a dose-finding study. Br J Anaesth 2007;98:598-603. [Crossref] [PubMed]
- Glass PS, Gan TJ, Howell S. A review of the pharmacokinetics and pharmacodynamics of remifentanil. Anesth Analg 1999;89:S7-14. [Crossref] [PubMed]
- Nho JS, Lee SY, Kang JM, et al. Effects of maintaining a remifentanil infusion on the recovery profiles during emergence from anaesthesia and tracheal extubation. Br J Anaesth 2009;103:817-21. [Crossref] [PubMed]
- Lee JH, Koo BN, Jeong JJ, et al. Differential effects of lidocaine and remifentanil on response to the tracheal tube during emergence from general anaesthesia. Br J Anaesth 2011;106:410-5. [Crossref] [PubMed]
- Cheng SS, Yeh J, Flood P. Anesthesia matters: patients anesthetized with propofol have less postoperative pain than those anesthetized with isoflurane. Anesth Analg 2008;106:264-9. table of contents. [Crossref] [PubMed]
- Irwin MG, Schraag S. NAP5 and depth of anaesthesia monitoring. Anaesthesia 2013;68:973-4. [Crossref] [PubMed]
- Pandit JJ, Cook TM. National Institute for Clinical Excellence guidance on measuring depth of anaesthesia: limitations of EEG-based technology. Br J Anaesth 2013;110:325-8. [Crossref] [PubMed]
- Pasetto A, Rinaldi L. Total Intravenous Anesthesia and Respiratory System. In: Gullo A, Berlot G, Lucangelo U, et al., editors. Perioperative and Critical Care Medicine. Milan: Springer-Verlag Italia, 2004:113-21.
- Protopapas AD, Athanasiou T. Peri-operative data on the Nuss procedure in children with pectus excavatum: independent survey of the first 20 years' data. J Cardiothorac Surg 2008;3:40. [Crossref] [PubMed]
- Pilegaard HK, Grosen K. Postoperative pain location following the Nuss procedure--what is the evidence and does it make a difference? Eur J Cardiothorac Surg 2010;38:208-9. [Crossref] [PubMed]
- Walaszczyk M, Knapik P, Misiolek H, et al. Epidural and opioid analgesia following the Nuss procedure. Med Sci Monit 2011;17:PH81-86. [Crossref] [PubMed]
- Pilegaard HK, Licht PB. Early results following the Nuss operation for pectus excavatum--a single-institution experience of 383 patients. Interact Cardiovasc Thorac Surg 2008;7:54-7. [Crossref] [PubMed]
- St Peter SD, Weesner KA, Sharp RJ, et al. Is epidural anesthesia truly the best pain management strategy after minimally invasive pectus excavatum repair? J Pediatr Surg 2008;43:79-82; discussion 82. [Crossref] [PubMed]
- Grosen K, Pfeiffer-Jensen M, Pilegaard HK. Postoperative consumption of opioid analgesics following correction of pectus excavatum is influenced by pectus severity: a single-centre study of 236 patients undergoing minimally invasive correction of pectus excavatum. Eur J Cardiothorac Surg 2010;37:833-9. [Crossref] [PubMed]
- Nagasao T, Miyamoto J, Ichihara K, et al. Age-related change of postoperative pain location after Nuss procedure for pectus excavatum. Eur J Cardiothorac Surg 2010;38:203-8. [Crossref] [PubMed]
- Futagawa K, Suwa I, Okuda T, et al. Anesthetic management for the minimally invasive Nuss procedure in 21 patients with pectus excavatum. J Anesth 2006;20:48-50. [Crossref] [PubMed]
- McBride WJ, Dicker R, Abajian JC, et al. Continuous thoracic epidural infusions for postoperative analgesia after pectus deformity repair. J Pediatr Surg 1996;31:105-7; discussion 107-8. [Crossref] [PubMed]
- Weber T, Mätzl J, Rokitansky A, et al. Superior postoperative pain relief with thoracic epidural analgesia versus intravenous patient-controlled analgesia after minimally invasive pectus excavatum repair. J Thorac Cardiovasc Surg 2007;134:865-70. [Crossref] [PubMed]
- Croitoru DP, Kelly RE Jr, Goretsky MJ, et al. Experience and modification update for the minimally invasive Nuss technique for pectus excavatum repair in 303 patients. J Pediatr Surg 2002;37:437-45. [Crossref] [PubMed]
- Soliman IE, Apuya JS, Fertal KM, et al. Intravenous versus epidural analgesia after surgical repair of pectus excavatum. Am J Ther 2009;16:398-403. [Crossref] [PubMed]
- Yegin A, Erdogan A, Kayacan N, et al. Early postoperative pain management after thoracic surgery; pre- and postoperative versus postoperative epidural analgesia: a randomised study. Eur J Cardiothorac Surg 2003;24:420-4. [Crossref] [PubMed]
- Hermanides J, Hollmann MW, Stevens MF, et al. Failed epidural: causes and management. Br J Anaesth 2012;109:144-54. [Crossref] [PubMed]
- Cook TM, Counsell D, Wildsmith JA, et al. Major complications of central neuraxial block: report on the Third National Audit Project of the Royal College of Anaesthetists. Br J Anaesth 2009;102:179-90. [Crossref] [PubMed]
- Behera BK, Puri GD, Ghai B. Patient-controlled epidural analgesia with fentanyl and bupivacaine provides better analgesia than intravenous morphine patient-controlled analgesia for early thoracotomy pain. J Postgrad Med 2008;54:86-90. [Crossref] [PubMed]
- Tuncel G, Ozalp G, Savli S, et al. Epidural ropivacaine or sufentanil-ropivacaine infusions for post-thoracotomy pain. Eur J Cardiothorac Surg 2005;28:375-9. [Crossref] [PubMed]
- Yapici D, Atici S, Alic M, et al. Morphine added to local anaesthetic improves epidural analgesia in minimally invasive Nuss operation for pectus excavatum. Br J Anaesth 2008;100:280. [Crossref] [PubMed]
- Cucchiaro G, Adzick SN, Rose JB, et al. A comparison of epidural bupivacaine-fentanyl and bupivacaine-clonidine in children undergoing the Nuss procedure. Anesth Analg 2006;103:322-7. table of contents. [Crossref] [PubMed]
- Fortier S, Hanna HA, Bernard A, et al. Comparison between systemic analgesia, continuous wound catheter analgesia and continuous thoracic paravertebral block: a randomised, controlled trial of postthoracotomy pain management. Eur J Anaesthesiol 2012;29:524-30. [Crossref] [PubMed]
- Hall Burton DM, Boretsky KR. A comparison of paravertebral nerve block catheters and thoracic epidural catheters for postoperative analgesia following the Nuss procedure for pectus excavatum repair. Paediatr Anaesth 2014;24:516-20. [Crossref] [PubMed]
- Lönnqvist PA. Continuous paravertebral block in children. Initial experience. Anaesthesia 1992;47:607-9. [Crossref] [PubMed]
- Karmakar MK. Thoracic paravertebral block. Anesthesiology 2001;95:771-80. [Crossref] [PubMed]
- Richardson J, Lönnqvist PA, Naja Z. Bilateral thoracic paravertebral block: potential and practice. Br J Anaesth 2011;106:164-71. [Crossref] [PubMed]
- Inge TH, Owings E, Blewett CJ, et al. Reduced hospitalization cost for patients with pectus excavatum treated using minimally invasive surgery. Surg Endosc 2003;17:1609-13. [Crossref] [PubMed]
- Rugyte DC, Kilda A, Karbonskiene A, et al. Systemic postoperative pain management following minimally invasive pectus excavatum repair in children and adolescents: a retrospective comparison of intravenous patient-controlled analgesia and continuous infusion with morphine. Pediatr Surg Int 2010;26:665-9. [Crossref] [PubMed]
- Lukosiene L, Rugyte DC, Macas A, et al. Postoperative pain management in pediatric patients undergoing minimally invasive repair of pectus excavatum: the role of intercostal block. J Pediatr Surg 2013;48:2425-30. [Crossref] [PubMed]
- Mavi J, Moore DL. Anesthesia and analgesia for pectus excavatum surgery. Anesthesiol Clin 2014;32:175-84. [Crossref] [PubMed]
- Li G, Jiang Z, Xiao H, et al. A novel modified Nuss procedure for pectus excavatum: a new steel bar. Ann Thorac Surg 2015;99:1788-92. [Crossref] [PubMed]
- Scott NB. Wound infiltration for surgery. Anaesthesia 2010;65 Suppl 1:67-75. [Crossref] [PubMed]
- Blanco R. The 'pecs block': a novel technique for providing analgesia after breast surgery. Anaesthesia 2011;66:847-8. [Crossref] [PubMed]
- Pérez MF, Miguel JG, de la Torre PA. A new approach to pectoralis block. Anaesthesia 2013;68:430. [Crossref] [PubMed]
- Sopena-Zubiria LA, Fernández-Meré LA, Valdés Arias C, et al. Thoracic paravertebral block compared to thoracic paravertebral block plus pectoral nerve block in reconstructive breast surgery. Rev Esp Anestesiol Reanim 2012;59:12-7. [Crossref] [PubMed]
- Bashandy GM, Abbas DN. Pectoral nerves I and II blocks in multimodal analgesia for breast cancer surgery: a randomized clinical trial. Reg Anesth Pain Med 2015;40:68-74. [Crossref] [PubMed]
- Blanco R, Parras T, McDonnell JG, et al. Serratus plane block: a novel ultrasound-guided thoracic wall nerve block. Anaesthesia 2013;68:1107-13. [Crossref] [PubMed]
- Liu T, Liu H, Yang C, et al. Brachial plexus palsy, a rare delayed complication of the Nuss procedure for pectus excavatum: a case report. J Pediatr Surg 2012;47:e19-20. [Crossref] [PubMed]
- Fox ME, Bensard DD, Roaten JB, et al. Positioning for the Nuss procedure: avoiding brachial plexus injury. Paediatr Anaesth 2005;15:1067-71. [PubMed]
- Umuroglu T, Bostancı K, Thomas DT, et al. Perioperative anesthetic and surgical complications of the Nuss procedure. J Cardiothorac Vasc Anesth 2013;27:436-40. [Crossref] [PubMed]
- Nuss D, Croitoru DP, Kelly RE Jr, et al. Review and discussion of the complications of minimally invasive pectus excavatum repair. Eur J Pediatr Surg 2002;12:230-4. [Crossref] [PubMed]
- Park HJ, Lee SY, Lee CS. Complications associated with the Nuss procedure: analysis of risk factors and suggested measures for prevention of complications. J Pediatr Surg 2004;39:391-5; discussion 391-5. [Crossref] [PubMed]
- Nath DS, Wells WJ, Reemtsen BL. Mechanical occlusion of the inferior vena cava: an unusual complication after repair of pectus excavatum using the nuss procedure. Ann Thorac Surg 2008;85:1796-8. [Crossref] [PubMed]
- Ballouhey Q, Léobon B, Trinchéro JF, et al. Mechanical occlusion of the inferior vena cava: an early complication after repair of pectus excavatum using the Nuss procedure. J Pediatr Surg 2012;47:e1-3. [Crossref] [PubMed]
- Hoel TN, Rein KA, Svennevig JL. A life-threatening complication of the Nuss procedure for pectus excavatum. Ann Thorac Surg 2006;81:370-2. [Crossref] [PubMed]
- Jemielity M, Pawlak K, Piwkowski C, et al. Life-threatening aortic hemorrhage during pectus bar removal. Ann Thorac Surg 2011;91:593-5. [Crossref] [PubMed]
- Cha MH, Eom JH, Lee YS, et al. Beneficial effects of adding ketamine to intravenous patient-controlled analgesia with fentanyl after the Nuss procedure in pediatric patients. Yonsei Med J 2012;53:427-32. [Crossref] [PubMed]
- Jin F, Chung F. Multimodal analgesia for postoperative pain control. J Clin Anesth 2001;13:524-39. [Crossref] [PubMed]
- Scheit MW, Litz RJ, Gaebler R, et al. Postoperative Thoracic Epidural Analgesia in Young Adolescents Undergoing Nuss' Procedure for Pectus Excavatum Repair. Anesthesiology 2005;103:A1387.
- Wildgaard K, Ravn J, Kehlet H. Chronic post-thoracotomy pain: a critical review of pathogenic mechanisms and strategies for prevention. Eur J Cardiothorac Surg 2009;36:170-80. [Crossref] [PubMed]
- Sihoe AD, Lee TW, Wan IY, et al. The use of gabapentin for post-operative and post-traumatic pain in thoracic surgery patients. Eur J Cardiothorac Surg 2006;29:795-9. [Crossref] [PubMed]
- Solak O, Metin M, Esme H, et al. Effectiveness of gabapentin in the treatment of chronic post-thoracotomy pain. Eur J Cardiothorac Surg 2007;32:9-12. [Crossref] [PubMed]
- Jaroszewski D, Johnson K, Lackey J, et al. Complex repair of pectus excavatum recurrence and massive chest wall defect and lung herniation after prior open repair. Ann Thorac Surg 2013;96:e29-31. [Crossref] [PubMed]
- Dilley AV, Cloyd H, Glass NL, et al. Pain Management after Minimally Invasive Pectus Excavatum Repair. Pediatric Endosurgery & Innovative Techniques 2004;5:163-7. [Crossref]
- Castellani C, Schalamon J, Saxena AK, et al. Early complications of the Nuss procedure for pectus excavatum: a prospective study. Pediatr Surg Int 2008;24:659-66. [Crossref] [PubMed]
- Maggiore SM, Idone FA, Vaschetto R, et al. Nasal high-flow versus Venturi mask oxygen therapy after extubation. Effects on oxygenation, comfort, and clinical outcome. Am J Respir Crit Care Med 2014;190:282-8. [Crossref] [PubMed]
- Parke R, McGuinness S, Dixon R, et al. Open-label, phase II study of routine high-flow nasal oxygen therapy in cardiac surgical patients. Br J Anaesth 2013;111:925-31. [Crossref] [PubMed]
- Kindgen-Milles D, Müller E, Buhl R, et al. Nasal-continuous positive airway pressure reduces pulmonary morbidity and length of hospital stay following thoracoabdominal aortic surgery. Chest. 2005;128:821-8. [Crossref] [PubMed]
- Oxford Heart & Lung Centre. Pectus Correction surgery. Information for patients. Available online: http://www.ouh.nhs.uk/patient-guide/leaflets/files%5C121105pectuscorrection.pdf
Cite this article as: Patvardhan C, Martinez G. Anaesthetic considerations for pectus repair surgery. J Vis Surg 2016;2:76.


