Revision of failed, recurrent or complicated pectus excavatum after Nuss, Ravitch or cardiac surgery
Introduction
The two most common methods used to repair pectus excavatum (PE) deformities include modifications of the open Ravitch approach and the minimally invasive repair of pectus excavatum (MIRPE) or “Nuss”. Recurrence rates after repair of PE using both techniques have been reported in 2–37% of patients (1-5). There are no scientific publications comparing long-term recurrences of MIRPE to open repair. The cause of recurrence is variable and based on the technique of initial repair.
Recurrence after primary MIRPE
Failure or recurrence after primary MIRPE repair is generally due to technical issues and includes (2,5-8):
Bar displacement:
- Bars placed too lateral;
- Bars too long;
- Bar stabilization/securing inadequate.
Failure to elevate sternum:
- Chest wall too stiff;
- Inadequate number of bars to support chest wall;
- Intercostal muscle stripping with lateral migration of bars.
The majority of centers experienced with revision of prior failed or recurrent MIRPE patients found that malpositioned or displaced bars were a large portion of the issue (5,7). Bars that were too long or placed too lateral were found to be a cause of the majority of Nuss failures in several publications (5,7). Intercostal stripping and lateral displacement can occur after placement and when occurs, the bar will fail to contact the sternum and support it anteriorly (Figure 1). Use of a different interspace was recommended should intercostal stripping and lateral displacement occur. Medial stabilizer placement, “claw” and figure of eight suture reinforcement of the ribs bordering the stripped intercostal space can also be performed (9). The utilization of forced sternal elevation may also help facilitate bar placement and rotation and minimize intercostal stripping (10,11).
Adequate stability is also impacted by the number of bars and balance of the chest wall on support structures. The pressure required to elevate the chest is significant and an inadequate number of bars to support the chest anterior can lead to lateral stripping of the intercostal and increased risk of bar rotation (8,10,12,13). Recommendations as to what is an adequate number of bars varies (2,14-16). Older patients have been reported to require more bars for PE repair and two or more bars may give better and more stable results (2,14-16). Recurrence has also been attributed to premature removal of the pectus bars before adequate remodeling has occurred and the chest wall secured into a corrected position. The optimal length of time recommended to leave support bars in place varies however, several experienced centers have increased their recommended time to 2–3 years (2,13,15). Patients with Marfan’s and other connective tissue disorders have been shown to have a higher risk for recurrence and recommendations are for leaving the bars in place for up to 4 years (1,2).
A significant problem encountered after a failed Nuss can be extensive intrathoracic “toxic” adhesions (7,17). These can require several hours of extensive adhesiolysis before dissection across the chest and mediastinum is achieved for bar placement. Use of sternal elevation may be helpful and others have described a subxiphoid incision to manually elevate the sternum during dissection across the chest, especially with extensive adhesions (7,17,18).
Recurrence after Ravitch or open repair
Modifications of the original technique described by Ravitch have been used for decades (18). Risk for recurrence is often related to incomplete healing and complications and includes the following:
- Incomplete excision of involved cartilages;
- Dissection too extensive or failure to preserve perichondrium on excised cartilage;
- Incomplete healing or failed fusion of excised cartilage and sternum;
- Infection and seroma complications;
- Failure to support repair or too early removal of support.
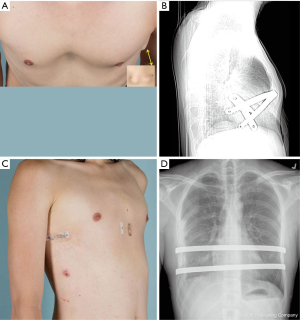
Once recurrence occurs, subsequent repair can be more complex due to chest wall rigidity and scar tissue from the prior surgical intervention. Extensive calcification, ossification and fusion of the previously excised cartilage may prevent adequate elevation of the chest wall without reexcision or osteotomy (18-20). Rigidity of the chest may make repair with MIRPE difficult and bar displacement may be a higher risk. Recurrences following open repair can also be associated with osteonecrosis, malunion and chest wall hernia (Figure 2) (1,6,18,20,21). Open repair and stabilization is recommended for these types of complex issues (18,22).
PE repair after prior cardiac surgery and sternotomy
Patients that have had prior sternotomy and cardiotomy may have significant adhesions to the posterior sternum. If pericardium was not closed at the time of cardiac procedure, the right atrium or ventricle may be adherent and attempts to dissect can lead to cardiac perforation and life threatening bleeding. Additional risks are present with reoperation with the majority of catastrophic events occurring during dissection of mediastinal adhesions (2,6,17). Cardiopulmonary bypass and ability to perform emergent sternotomy should be planned if necessary.
There are a limited number of publications describing repair of recurrent pectus deformities (1,5-7,17,18,21) and generally experience with a single operative technique is discussed for repair of the recurrence. Most publications note the need for multiple bars and slightly higher complications and bar displacement rates with revisions. Others have advocated the use of a modified open Ravitch repair in all patients with recurrent PE (18). Repairs in adult patients may be more difficult and have increased risks of complications due to increased rigidity of the chest wall as well as issues with healing (4,14,21,23). Complex open repairs were required in many adult patients after prior open repair when compared to other studies (18-20). In our own experience of repairs after recurrent open procedures, a hybrid technique was utilized to optimize repair in over 70% of patients (22). Follow up on all studies is limited and the long-term durability of repairs unknown.
Patient selection and workup
Indications for repair of recurrent PE are similar to those for primary repair and include Haller Index greater than 3.25 or Correction Index greater than 20% (5,6,17,18,21), evidence for cardiac compression and symptomology correlating with return of defect. Additionally, patients that have undergone previous open repair may have areas of non-union, chest wall hernia or other conditions that lead to chronic pain and chest wall instability (20,21). Symptoms resulting from these type issues can be severe and may be an indication for surgery despite not meeting measurement criteria. Great consideration should be given to reoperation given the increased operative complexity and risk of complications. Patients need to be educated about increased surgical complications, recovery period, and have realistic expectations of final results.
A recommended work up for revision cases includes the following:
- Physical exam: assessment of compliance and residual flexibility of anterior chest wall. Identification of areas with pseudoarthrosis or malunion between the sternum and ribs (24);
- Computerized tomography or magnetic resonance imaging: measurement of the extent of PE defect and visualization of areas of malunion or non-union that may not be identified on physical exam. Identification of chest wall hernia, irregular cartilage regeneration at the retrosternal level and incomplete reunion of previous resection sites can be performed;
- Assessment of prior procedures performed (operative notes when available), and any information regarding postoperative complications and recurrence;
- Evaluation of physiologic abnormalities which may include echocardiogram, pulmonary functions and cardiopulmonary V02 and exercise parameters (2,3).
Equipment preference card
Positioning equipment: Jelly rolls ×2 lengthwise under back, supine with pillow under knees, secure arms by folding draw sheet over arms and tuck under patient.
Prep: Chloraprep (CareFusion Corp, San Diego, CA, USA) and Ioban (3M, St. Paul, MN, USA).
Suture: #5 FiberWire® (Arthrex Inc., Naples, FL, USA), absorbable Vicryl for subcutaneous and skin.
Equipment: Olympus 5-mm Endoeye Flex 5, video cart and accessory monitor; 5 mm thoracoscopic ports ×2, Rultract Retractor (Rultract Inc., Cleveland, OH, USA); Lewin Bone Clamp (Lewin Spinal Perforating Forceps, V. Mueller NL6960; CareFusion, Inc., San Diego, Calif, USA); BioMet Pectus kit (BioMet Zimmer, Jacksonville, FL, USA); 1 and 2 inch osteotomes.
Implantables: Synthes Titanium Sternal Fixation System (DePuy Synthes, West Chester, PA, USA); Strattice Biomesh (Strattice Reconstructive Tissue Matric LifeCell Corp, Bridgewater, NJ, USA); Cadaveric Bone Graft (Medtronic Sofamor Danek Putty); osteotomes & mallet; perichondrial elevator; On-Q disposable 17-gauge ×8-inch tunneling system (Model T17X8, Halyard Health); 7.5-cm soaker catheters (MP050-A, Halyard Health).
On-Q pump with Select-A-Flow Variable Rate Controller (On-Q pump, Halyard Health.com, Irvine, CA, USA) catheter infusing Ropivacaine 0.2 mg. ON-Q catheters were primed and attached to a 750 mL reservoir.
Anesthesia and analgesia
Perioperative: all patients are given in the pre-operative morning the following medications:
- Gabapentin 600 mg oral;
- Celecoxib 400 mg oral;
- Acetaminophen 1,000 mg oral;
- Clonidine transdermal patch 0.1 mg q 72 hours;
- Patients receive either a thoracic epidural analgesia (TEA) or subcutaneous On-Q catheters (ONQ). In the TEA group, a thoracic catheter was inserted by an attending cardiothoracic anesthesiologist and an epidural infusion of ropivacaine, 0.2%, was started at 4–6 mL/h during the surgical procedure. Epidural catheters were left in place for 48 hours and removed on postoperative day 2.
Intraoperative:
- General anesthesia induced with intravenous fentanyl 0.5–2 mcg/kg, and propofol 2–4 mg/kg;
- Methadone 0.2–0.35 mg/kg intravenous;
- Ketorolac 30 mg intravenous;
- Patients who chose the On-Q arm had multi-holed, 7.5-cm wound catheters inserted by the thoracic surgeon at the end of the surgical procedure. The catheters were tunneled bilaterally in the subcutaneous tissues of the axilla, lateral to the surgical site. Each catheter infused ropivacaine hydrochloride, 0.2% and was locked at a rate of 7 mL/h.
Procedures
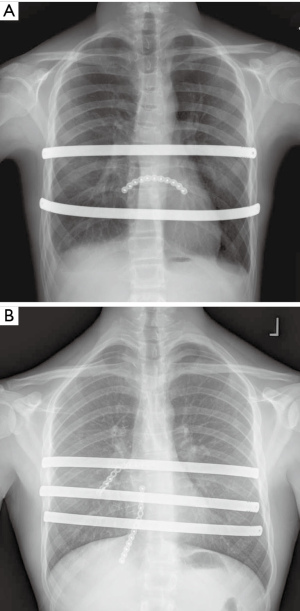
All patients are administered intravenous antibiotic prophylaxis (Cefazolin 1–2 grams IV unless allergic) prior to initiation of procedure. General anesthesia with double-lumen intubation is performed. The patient is positioned supine with arms secured at the sides. Gel rolls are placed under the back parallel to the spine and the arms padded and tucked at the sides. Groins are left exposed and prepped into the surgical field should emergent access and cardiopulmonary bypass be necessary (Figure 3). This positioning facilitates access to both anterior and lateral aspects of the chest wall for placing and affixing bars. ChloraPrep (CareFusion Corp, San Diego, CA, USA) and Ioban (3M, St. Paul, MN, USA) are utilized.
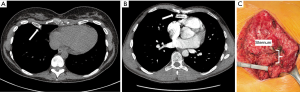
For the majority of reoperative patients, a modified MIRPE utilizing forced elevation is utilized (10). Figure 4 is an algorithm for our approach to revision patients after MIRPE and open procedures. Open resection with osteotomy and partial modified revision Ravitch are performed when necessary if the chest wall will not elevate adequately. Patients with pseudoarthrosis or “floating sternum” are planned for a combined procedure with elevation of the chest wall and stabilization of sternocostal instability (24). For patients with prior sternotomy or cardiac surgery, dissection of the mediastinum can lead to catastrophic bleeding.
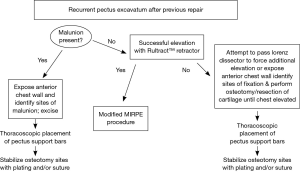
Patients with prior sternotomy are assessed for risk of adhesions of the epicardium to the sternum. If the risk is thought to be minimal, thoracoscopic dissection of the mediastinum is attempted with cardiopulmonary bypass on standby. After successful dissection, a modified MIRPE is performed as described. Patients with high risks of cardiac adhesions may require a redo sternotomy prior to attempting PE repair. The mediastinal adhesions are dissected free and the sternum closed with interrupted sternal wires. With severe PE deformity, the sternal edges may need to be cut with an oscillating saw to create an angle which allows tight approximation. Thoracoscopic placement of support bars and MIRPE is then performed after closure of the sternum.
Modified MIRPE
A 3-cm incision is made bilaterally following the rib contour at the inferolateral pectoral borders. The pectoralis muscles are elevated off the chest wall along the anterior and lateral chest wall utilizing cautery. Initially a 5 mm port is placed through the right incision and carbon dioxide insufflation utilized as well as single-lung ventilation. Under thoracoscopic visualization, a second 5 mm port is placed inferiorly above the right chest diaphragm and the camera moved to this location. Intrathoracic adhesions are taken down with a combination of cautery and blunt dissection. The mediastinum is not dissected until sternal elevation is achieved. Incisions are placed on either side of the sternum at an interspace and a perforating bone clamp inserted into the anterior table of the sternum. The Rultract Retractor is attached to the table at the level of the mid-sternum on the left side. The sternum is then attempted for elevation (Figure 5).
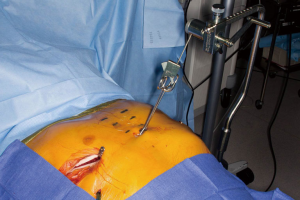
If elevation is achieved, a modified MIRPE will be performed
Procedure for modified MIRPE
Multiple bars are guided into position in the interspaces spanning the defect. Bars are flipped into place with the sternum still held elevated to minimize stresses to the intercostal space. Multipoint circumferential fixation of the bars and ribs is performed bilateral using FiberWire® (Arthrex Inc., Naples, FL, USA) (25).
If forced sternal elevation cannot elevate the chest anteriorly or malunion and sternal floating evident a hybrid repair is performed (22)
Hybrid procedure after recurrent PE (Figure 6)

If forced sternal elevation fails, an attempt with the Lorentz dissector can also be made. If the chest does not elevate properly or chest wall instability evident, the midline incision from patient’s previous procedure is opened and dissection taken down to the bony chest wall. Sites of fixation or malunion are identified. Osteotomy of the sternum and identified improperly positioned/fused ribs is performed where necessary using bone chisels or a powered bone saw.
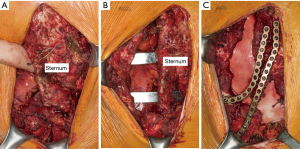
Once chest mobility is obtained, anterior elevation with the Rultract is obtained. Exploration and takedown of the mediastinum is thoracoscopically performed. In cases with significant pericardial adhesions to the sternum, addition of a subxiphoid approach has been advocated by some (7,17). Once the dissection is complete, placement and securing of support bars is performed as previously described (Figure 7). Attention is then returned to the open chest wall. Stabilization of osteotomies of the sternum and sterno-costal junctions may be necessary. Repeated osteotomies in similar locations may be prone to malunion or non-union, therefore, titanium sternal plating and FiberWire fixation are used to approximate the sites of costocartilage/rib to the sternum following elevation (Figure 8). For deformities with extensive osteonecrosis and chest wall hernia, use of cadaveric bone graft, methylmethacrylate and biologic mesh can be utilized for repair (19,20) (Figure 9).
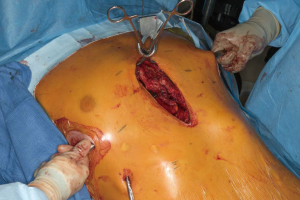
The pectoralis and rectus abdominus muscles are reattached to the chest wall and subcutaneous tissues and skin closed with layered absorbable suture. Medium drains are left under the pectoralis muscles to prevent seroma accumulation. Chest tubes are placed through the lower port site on the right and left if deemed necessary.
For patients receiving On-Q catheters, the surgeon tunnels 7.5-cm soaker catheters bilaterally in the subcutaneous tissues in the anterior axilla (Figure 10). All patients additionally had a bilateral intercostal block [bupivacaine hydrochloride, 0.25% (1 mL/kg)] and received intravenous ketorolac, 30 mg, and ondansetron, 4 mg at completion of the surgical procedure.
Postoperative management
Postoperative patients are kept on telemetry monitoring for 24 hours. Discharge is planned when chest tubes are out and patient pain is controlled on oral medications. Chest tubes are removed when output is less than 300 mL for 24 hours. If subpectoral drains are placed, removal occurs when output less than 30–40 mL for 24 hours and often patients are discharged home on antibiotic prophylaxis with drains still in place. Postoperative pain management in addition to epidural or On-Q catheters is as follows:
Postoperative 24 hours:
- Patient controlled analgesia (PCA) with 0.2 mg Dilaudid at 8 minutes interval dosing with a 4.8 mg lockout at 4 hours (increase in on demand dosing up to 0.4 mg allowed if necessary);
- Ketorolac 30 mg intravenous every 8 hours;
- Gabapentin 300 mg oral every 8 hours;
- Acetaminophen 1,000 mg oral every 6 hours;<//
- Clonidine transdermal patch 0.1 mg every 72 hours.
Postoperative 24–48 hours and discharge home:
- PCA discontinued morning of postoperative day 1;
- OxyContin 10–20 mg orally every 12 hours initiated;
- Oxycodone 5–10 mg orally every 2–4 hours as needed;
- Ketorolac discontinued and ibuprofen 600 mg every 8 hours initiated;
- Gabapentin 300 mg oral every 8 hours;
- Acetaminophen 1,000 mg every 6 hours;
- Clonidine transdermal patch 0.1 mg every 72 hours.
The On-Q catheters are primed and attached to a 750-mL, fill-volume reservoir, which is refilled after 48 hours. The catheters were then left in place for a maximum of 7 days. Patients are discharged home with the On-Q pump system unless they asked to have it removed.
Tips, tricks and pitfalls
Surgical repair of recurrent PE can be complex and presents special challenges. The type of initial repair impacts successful revision. Patients with malunion and significant ossification and deformity should not be considered as candidates for MIRPE alone and the hybrid procedure may be a good option. Adequate anterior elevation of the chest must be obtained before support bar placement and reinforcement of interspaces should be performed for difficult elevations. Osteotomy of the sternum or sternocostal junctions should be performed as necessary to allow sternal elevation when fixed and inflexible. Once elevation of the chest wall is achieved, complete stabilization of the freed chest wall segments in the desired anatomic position should be performed.
Significant mediastinal scarring and pleural adhesions may be encountered in revision procedures. Positioning and draping to allow rapid emergent access to femoral vessels and availability of cardiopulmonary bypass are important for complex reoperative patients. The use of forced sternal elevation or subxiphoid dissection has been recommended by others to increase the safety of dissecting mediastinal adhesions (7,10,13,17).
Conclusions
Repair of recurrent PE after prior open PE surgery can be difficult. A variety of techniques can be successful and understanding the cause of initial recurrence is critical. With appropriate techniques, PE recurrences can be repaired with good results and outcomes.
Acknowledgements
None.
Footnote
Conflicts of Interest: Dr. Jaroszewski reports personal fees from Zimmer BioMet, outside the submitted work.
References
- Ellis DG, Snyder CL, Mann CM. The 're-do' chest wall deformity correction. J Pediatr Surg 1997;32:1267-71. [Crossref] [PubMed]
- Kelly RE, Goretsky MJ, Obermeyer R, et al. Twenty-one years of experience with minimally invasive repair of pectus excavatum by the Nuss procedure in 1215 patients. Ann Surg 2010;252:1072-81. [Crossref] [PubMed]
- Kelly RE Jr, Mellins RB, Shamberger RC, et al. Multicenter study of pectus excavatum, final report: complications, static/exercise pulmonary function, and anatomic outcomes. J Am Coll Surg 2013;217:1080-9. [Crossref] [PubMed]
- Mansour KA, Thourani VH, Odessey EA, et al. Thirty-year experience with repair of pectus deformities in adults. Ann Thorac Surg 2003;76:391-5; discussion 395. [Crossref] [PubMed]
- Croitoru DP, Kelly RE Jr, Goretsky MJ, et al. The minimally invasive Nuss technique for recurrent or failed pectus excavatum repair in 50 patients. J Pediatr Surg 2005;40:181-6; discussion 186-7. [Crossref] [PubMed]
- De Ugarte DA, Choi E, Fonkalsrud EW. Repair of recurrent pectus deformities. Am Surg 2002;68:1075-9. [PubMed]
- Redlinger RE Jr, Kelly RE Jr, Nuss D, et al. One hundred patients with recurrent pectus excavatum repaired via the minimally invasive Nuss technique--effective in most regardless of initial operative approach. J Pediatr Surg 2011;46:1177-81. [Crossref] [PubMed]
- Park HJ, Chung WJ, Lee IS, et al. Mechanism of bar displacement and corresponding bar fixation techniques in minimally invasive repair of pectus excavatum. J Pediatr Surg 2008;43:74-8. [Crossref] [PubMed]
- Park HJ, Kim KS, Lee S, et al. A next-generation pectus excavatum repair technique: new devices make a difference. Ann Thorac Surg 2015;99:455-61. [Crossref] [PubMed]
- Jaroszewski DE, Johnson K, McMahon L, et al. Sternal elevation before passing bars: a technique for improving visualization and facilitating minimally invasive pectus excavatum repair in adult patients. J Thorac Cardiovasc Surg 2014;147:1093-5. [Crossref] [PubMed]
- Tedde ML, de Campos JR, Wihlm JM, et al. The Nuss procedure made safer: an effective and simple sternal elevation manoeuvre. Eur J Cardiothorac Surg 2012;42:890-1. [Crossref] [PubMed]
- Fonkalsrud EW, Reemtsen B. Force required to elevate the sternum of pectus excavatum patients. J Am Coll Surg 2002;195:575-7. [Crossref] [PubMed]
- Park HJ, Jeong JY, Jo WM, et al. Minimally invasive repair of pectus excavatum: a novel morphology-tailored, patient-specific approach. J Thorac Cardiovasc Surg 2010;139:379-86. [Crossref] [PubMed]
- Aronson DC, Bosgraaf RP, van der Horst C, et al. Nuss procedure: pediatric surgical solution for adults with pectus excavatum. World J Surg 2007;31:26-9; discussion 30. [Crossref] [PubMed]
- Nuss D. Minimally invasive surgical repair of pectus excavatum. Semin Pediatr Surg 2008;17:209-17. [Crossref] [PubMed]
- Pilegaard HK. Extending the use of Nuss procedure in patients older than 30 years. Eur J Cardiothorac Surg 2011;40:334-7. [PubMed]
- Guo L, Mei J, Ding F, et al. Modified Nuss procedure in the treatment of recurrent pectus excavatum after open repair. Interact Cardiovasc Thorac Surg 2013;17:258-62. [Crossref] [PubMed]
- Luu TD, Kogon BE, Force SD, et al. Surgery for recurrent pectus deformities. Ann Thorac Surg 2009;88:1627-31. [Crossref] [PubMed]
- Jaroszewski DE, Notrica DM, McMahon LE, et al. Operative management of acquired thoracic dystrophy in adults after open pectus excavatum repair. Ann Thorac Surg 2014;97:1764-70. [Crossref] [PubMed]
- Jaroszewski D, Johnson K, Lackey J, et al. Complex repair of pectus excavatum recurrence and massive chest wall defect and lung herniation after prior open repair. Ann Thorac Surg 2013;96:e29-31. [Crossref] [PubMed]
- Colombani PM. Recurrent chest wall anomalies. Semin Pediatr Surg 2003;12:94-9. [Crossref] [PubMed]
- Johnson KN, Jaroszewski DE, Ewais M, et al. Hybrid Technique for Repair of Recurrent Pectus Excavatum After Failed Open Repair. Ann Thorac Surg 2015;99:1936-43. [Crossref] [PubMed]
- Vegunta RK, Pacheco PE, Wallace LJ, et al. Complications associated with the Nuss procedure: continued evolution of the learning curve. Am J Surg 2008;195:313-6; discussion 316-7. [Crossref] [PubMed]
- Prabhakaran K, Paidas CN, Haller JA, et al. Management of a floating sternum after repair of pectus excavatum. J Pediatr Surg 2001;36:159-64. [Crossref] [PubMed]
- McMahon LE, Johnson KN, Jaroszewski DE, et al. Experience with FiberWire for pectus bar attachment. J Pediatr Surg 2014;49:1259-63. [Crossref] [PubMed]
- Jaroszewski DE, Ewais MM, Lackey JJ, et al. Procedure for hybrid PE and chest wall malunion on a 30-year-old with failed prior Ravitch. Asvide 2016;3:194. Available online: http://www.asvide.com/articles/950
Cite this article as: Jaroszewski DE, Ewais MM, Lackey JJ, Myers KM, Merritt MV, Stearns JD, Gaitan BD, Craner RC, Gotway MB, Naqvi TZ. Revision of failed, recurrent or complicated pectus excavatum after Nuss, Ravitch or cardiac surgery. J Vis Surg 2016;2:74.


