Irreversible electroporation of stage 3 locally advanced pancreatic cancer: optimal technique and outcomes
Introduction
It is estimated that of the 42,000 patients diagnosed with pancreatic adenocarcinoma 45% with have stage III (locally advanced) disease with involvement of the celiac axis or the superior mesenteric artery (SMA) (1,2). Past outcomes in these rare patients that are able to undergo resection with various systemic chemotherapies are poor: post-resection 5-year survival has been reported at 6.8% and the median survival after resection has been reported to be 10.6 months (3). This poor past prognosis has historically diminished enthusiasm for aggressive surgical resection (4).
Recent publications from Kwon et al. have reported superior overall survival with surgical resection with simultaneous irreversible electroporation (IRE) for margin accentuation in combination with active systemic chemotherapy and/or radiation therapy (5). IRE is a technique in which multiple (100 to 200) short (70 to 90 usec), high-voltage (1,500 volts/cm) pulses are applied to tissues (6-9) to permeabilize the cell membranes. IRE uses a nonthermal/electrical-based method of action and can be used to treat around vital structures such as the urethra, larger blood vessels, and nerves (7). Although irreversible electroporation for locally advanced pancreatic adenocarcinoma is a surgical palliative technique in locally advanced pancreatic disease, that has been reported and is currently standardized with the use of multiple needles (10). We have recently published our findings regarding the safety of IRE in the pancreas (11). Similarly we have also recently published superior survival rates with the use of IRE in combination with standard chemotherapy and/or chemo-radiation therapy when compared to standard of care chemotherapy or chemoradiation therapy (12).
This article describes our preferred method for the utilization of open irreversible electroporation of patients with locally advanced pancreatic adenocarcinoma.
Methods
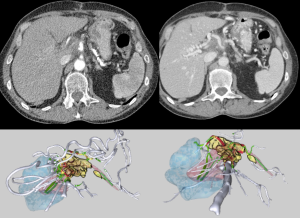
Our standard work-up for patients with locally advanced pancreatic adenocarcinoma includes a high quality 3-phase CT scan with pancreatic protocol with 0.7 mm cuts at the time of diagnosis, which allows us to appropriately diagnose and stage patients with locally advanced pancreatic adenocarcinoma. Additional 3 dimensional (3D) imaging is also performed of these patients to better document vessel involvement and proximity (Figure 1). We adhere to standard diagnostic criteria of stage III pancreatic cancer such that there must be greater than 180 degree encasement of the major arterial structures (superior mesenteric and/or celiac) without evidence of any type of metastatic disease to the liver or distant lymph nodes, nor any evidence of peritoneal disease (13,14). Laboratory work-up is also performed to ensure appropriate hematologic as well as CA19-9 evaluation. Following that a staging/diagnostic laparoscopy is performed at the time of diagnosis in which peritoneal washings are obtained, as well, to ensure small occult metastases are not present that have not been visualized on CT scan. Only after this is performed do we embark on induction chemotherapy of either FOLFIRINOX-based chemotherapy (in younger patients approximately less than 75 years of age and without evidence of non-alcoholic steatohepatitis) or gemzar combination based therapy after a thorough discussion of patient’s physiologic age and performance status. The goal is for at least 3-4 months of induction based therapy (gemzar chemotherapy consists of: approximately 3-4 cycles of 2 weeks, on and 1 week off. FOLFIRINOX: is given for approximately 4-6 cycles). Following that induction chemotherapy, we repeat high-quality 3-phase CT scan, and also obtain hematologic and serologic markers to ensure locally advanced non-metastatic pancreatic adenocarcinoma still exists. The key goal of this repeat imaging is to ensure that metastatic disease has not occurred, since it is uncommon for a pancreatic cancer to truly respond or reduce in size during induction therapy (chemotherapy alone or chemo-radiation therapy) based on established RECIST criteria (i.e., reduction in size of >30% of the longest diameter). As long as the patient has not developed metastatic disease and the maximum axial diameter is not above 4 cm after induction therapy, then we do proceed with IRE therapy.
Once this is confirmed, approximately 2-3 weeks after the last dose of chemotherapy open IRE to the pancreatic tumor primary is performed. Optimal inclusion of patients who are appropriate for irreversible electroporation should include tumor sizes that are 3.0-3.5 cm in maximum diameter. Patients with metal biliary stents can be treated if that metal stent can be removed prior to or at the time of irreversible electroporation. It has been our experience that patients with the long uncovered or partially covered biliary stents are much more difficult to remove than the short (4 cm) fully covered biliary stent. In either case, removal of metal stents is critical to patient outcomes. Given that any type of metal is conductive, it has been demonstrated in our large animal model that these metal stents lead to significant deflection of energy, which can lead to incomplete ablation, high current conditions, and possible thermal injury since the degree of deflection is not consistent based on the location of the metal, the probe exposure and the fibrotic nature of the tissue to be electroporated. If metals stents are removed then a Roux-en-Y hepatico-jejunostomy is performed at the same procedure as the IRE. This procedure is performed through an open laparotomy; appropriate cardiac and pulmonary evaluation should be performed to ensure the patient can tolerate this type of procedure.
Protocol
Step 1: Upper midline incision from 4 cm below xiphoid process and to umbilicus—approximately 6-8 cm in length.
Step 2: Thorough exploration and placement of Thompson retractor using single blade underneath upper midline to lift and two bladder blades to retract midline incision.
Step 3: Ultrasound of liver to ensure no liver metastasis. Ultrasound via a transgastric technique to ensure locally advanced tumor not amenable to resection. Ultrasound of pancreatic tumor to assess 3D size (anterior-posterior, axial, and cranial-caudal planes).
Step 4: Confirm a trans-mesocolic approach optimal for lower based pancreatic head/uncinate process lesions versus mobilizing omentum and a direct pancreatic approach for superior based head lesion.
Step 5: Using continuous ultrasound at the tissue insertion site to ensure ATRAMAUTIC needle placement bracketing vital structures and tumor to insure an adequate margin.
Step 6: Using deep paralytic and adequate narcotic, IRE to all needle pairs of a total 20 pulses is delivered to assess tissue fibrosis and tissue resistance, followed by the remaining 100 pulses for efficacy.
Step 7: Confirmation of IRE efficacy through delivery of electroporation energy to verify a change in amperage draw of an amount to ensure that adequate electroporation has occurred.
Step 8: Confirmation of vital structure patency through repeat ultrasound using power Doppler imaging to confirm vital structure flow and patency.
Step 9: Consideration of prophylactic gastrojejunostomy, J-tube or hepaticojejunostomy at surgeon’s discretion.
Results
Operative description (Figure 2)

Abdominal approach (Table 1)

Full table
The patient undergoes standard endotracheal intubation and access for open IRE is performed through a superior midline incision. A superior midline incision is utilized based on the planned needle placement performed most commonly and, I believe, in a safer manner through a caudal-to-cranial approach. In turn, the caudal-to-cranial approach is more easily facilitated through a midline laparotomy than through a bilateral subcostal laparotomy. The abdomen is thoroughly examined to rule out any type of occult solid organ liver metastases as well as peritoneal or mesenteric metastases. Intraoperative ultrasound of the liver is also performed to rule out any type of non-palpable liver metastases that may have been missed on dynamic CT scan. Only after no evidence of metastatic disease is confirmed is intraoperative ultrasound (Figure 3, BK Medical Ultrasound System—Flex Focus 800, Peabody, MA) then turned to the operative assessment of the tumor. Given the lack of definitive accuracy as well as positive predictive value of CT scan alone because of volume averaging, it is important to ensure that the patient truly has greater than 180 degree encasement of the SMA before deciding on in situ IRE therapy vs. pancreaticoduodenectomy with margin accentuation with IRE along the SMA. Our optimal ultrasound technique is transgastric and is performed with placing the ultrasound probe on top of the gastric body closer to the pylorus. I recommend imaging with minimal amount of mobilization and avoiding the mobilization into the lesser sac, which further impedes optimal intraoperative imaging since this will disrupt the tissue planes with air and lead to a greater artifact. The reason for performing through a transgastric approach (Figure 4) is that the stomach serosa allows for a complete and clean apposition of the ultrasound crystals and provides minimal to no artifact to truly image a pancreatic head lesion and subsequent portal vein SMA as well as superior mesenteric vein (SMV). I have found this transgastric approach is also the most sensitive way to assess invasion of the SMA without the need for extensive dissection. Thus, intraoperative ultrasound imaging has become our gold standard for elucidating whether a patient has a true locally advanced tumor or a borderline resectable tumor.
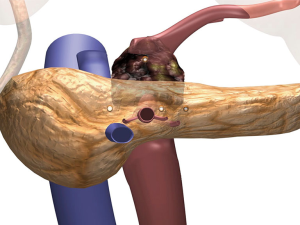
Once local advancement is confirmed and an in situ IRE is then planned, imaging of the tumor and the surrounding structures is then performed in order to obtain axial, anterior/posterior as well cranial/caudal maximum tumor diameters. Vital structures that need to be included in those diameters for appropriate needle placement are also assessed. Given that a majority of pancreatic tumors’ longest axis is in the cranio-caudal along the SMA for head lesions and on top of the SAM for neck lesions (approximately 4 cm), it is not uncommon to have a tumor that is longer in the cranio-caudal plane (approximately 3-4 cm) and the maximum axial diameter between 2.5 and 3.0 cm in size. Based on the maximum axial diameter appropriate needles are placed at exactly 2.0 cm apart so that the entire tumor and an approximate 1.0 cm margin of normal soft tissue is included in the IRE plane (Figure 5).
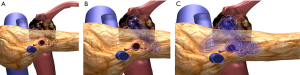
As demonstrated in Figure 5, this most commonly requires four needles in a trans-mesocolon approach, two to three needles posteriorly to cover the retro-peritoneum and then one or two anterior to cover the anterior extent of the tumor. One to two additional probes are then placed in a more anterior approach, most commonly 1.5 cm anteriorly such that a triangle or an oblong square is then obtained (Figure 5).
The optimal placement of the IRE needles is performed through continuous intraoperative ultrasound from the insertion of the needle into the tissue so that the needle tip is followed at all times during needle placement. I have found that placing these needles through the transverse mesentery, with care not to damage the transverse colon vessels, is easier because it allows normal soft tissue to bracket the pancreatic head tumor as well as to allow for appropriate inferior margin to be obtained during pullbacks of the needle. Thus, the transverse mesocolon is grasped and raised anteriorly out of the abdomen by an assistant and then the surgeon’s dominant hand directs the needle into the tissue, while her/his non-dominant hand utilizes the ultrasound probe to ensure accurate and appropriate needle placement. It cannot be overemphasized that an atraumatic needle placement should be performed to ensure that the needle does not damage the underlying vital structures, namely the SMV, portal vein, and SMA. Vascular needle trauma may induce underlying vascular thrombosis, especially given the potential hypercoagulable state in a patient with pancreas cancer.
We commonly will place the most lateral needle (probe 1—Figure 5) within the pancreatic at the most lateral extent. Then using spacers at 2.0 cm intervals we build off that initial needle to ensure adequate treatment margin(s). Once this margin(s) is obtained, one or two needles are then placed anterior in order to obtain complete bracketing of the tumor while allowing the normal non-tumor bearing tissue—that being the posterior aspect of the stomach anteriorly, the duodenum laterally and the transverse mesocolon inferiorly—to be left in place.
Care should also be undertaken that the maximum needle exposure to perform safe IRE of the pancreas should be 1.0 to 1.5 cm because of the significant fibrotic nature of these tumors and a larger needle exposure will not be tolerated by the gland or the underlying soft tissue to be treated. We have previously published that a greater probe exposure leads to high current conditions and the potential for thermal damage if these high current conditions are allowed to persist. Thus the maximum probe exposure should be 1.5 cm or less (16).
Following appropriate needle placement and ultrasound confirmation of appropriate spacing, those spacing measurements are entered into the energy unit’s software, which allow for optimal voltage and pulse length delivery. Standard default voltage of 1,500 volts/cm is initiated with planned delivery of 90 pulses and a pulse width of 70 to 90 usec. Twenty pulses are delivered initially and then the delivery is halted in order to assess the underlying amperage draw to establish optimal voltage and pulse widths. If the current amperage draw for these first 20 pulses is less than 35 amps I believe that this is an appropriate voltage per cm and pulse widths for safe and effective electroporation. Energy is delivered between all needle pairs (Figure 5) and evaluation of the energy delivered is then assessed for each pair in order to demonstrate a change in current amperage draw, which has been found to be an appropriate surrogate marker of change and resistance. This change in resistance is of utmost importance to ensure against reversible electroporation, which would lead to ineffective therapy and electroporation failure. Once effective current delivery has been confirmed between all pairs the needles are pulled back the appropriate distance such that no overlapping treatments are performed. Sequential pullbacks are performed in order to obtain adequate margins both superiorly and inferiorly. Each probe pair is (Figure 5) then treated again following subsequent pull back and again is re-treated for a total 180 pulses, or even in a rare instance 270 pulses if the current draw does not appropriately change over each 90 pulses delivery. Following optimal pulse delivery at each needle placement and providing appropriate margins are felt to be obtained with the needle placement, the needles are removed without the need for any additional hemostatic procedures (i.e., suture ligature, etc.) in most cases. Another probe configuration using a triangle formation is sometimes needed based on a width of the axial plane of the tumor that at times narrows anteriorly (Figure 5)
Because of the underlying tissue edema we have not had to do any specific surgical procedures to control needle site bleeding. At most, if needle placement has punctured one of the small transverse mesocolon vessels, a suture ligature is necessary. It should be noted that continuous intraoperative ultrasound is performed during all IRE delivery in order to assess energy delivery as well as to continually evaluate vascular patency if indeed the treating surgeon feels necessary.
Following treatment a prophylactic gastro-jejunostomy is commonly performed in conjunction with a jejunal feeding tube. An abdominal drain is usually not placed in patients who undergo just in situ IRE.
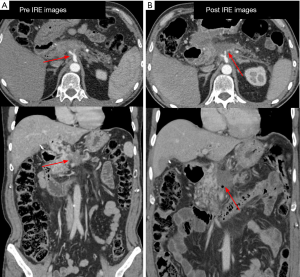
The postoperative management of these patients is fairly standard and follows guidelines for any type of pancreatic resection. The return of gastrointestinal function and the length of stay still remain approximately 6-10 days. An initial efficacy CT scan (Figure 6) is not obtained until 3 months post IRE because of the protracted method of action that occurs with IRE. Imaging prior to that will be inaccurate because of the edema and ongoing apoptosis, which is the most common method of IRE induced cell death as demonstrated in large porcine model experiments (16,17). Commonly, re-initiation of systemic chemotherapy is performed before this 3-month CT scan. A patient in whom external beam radiation therapy is felt necessary (i.e., to cover regional lymph nodes) is also initiated prior to this 3-month CT scan if the multidisciplinary team feels necessary.
Discussion
The initial evaluation of this device was first published in May 2012, in which 27 patients with unresectable pancreatic cancer underwent IRE. The group comprised 13 women and 14 men, with median age of 61 years (45-80 years of age). Eight patients underwent margin accentuation with IRE in combination with left-sided resection (n=4) or pancreatic head resection (n=4) with the goal to extend the margin-negative treatment. Nineteen patients had in situ IRE for locally advanced unresectable lesions in the head of the pancreas. All patients underwent successful IRE, with intra-operative imaging confirming effective delivery of therapy. All 27 patients demonstrated non-clinically relevant elevation of their amylase and lipase, which peaked at 48 hours and returned to normal at 72 hours post-procedure. There has been a 90-day mortality. No patient has shown evidence of clinical pancreatitis or fistula formation. After all patients have completed 90-day follow up there has been 100% ablation success (11). There was no evidence of intra-operative bleeding, no evidence of pancreatic fistula, no evidence of damage to surrounding viscera. This initial safety profile was then reproduced in a large cohort of 54 patients treated with IRE with a similar adverse event rate and specificity (12). A total of 54 locally advanced pancreatic cancer patients have successfully undergone IRE, a group comprising 21 women and 23 men with a median age of 61 years (45-80 years). These subjects were evaluated for overall survival and propensity matched to 85 matched stage III patients treated with standard therapy, defined as chemotherapy and radiation therapy alone. Thirty-five patients had pancreatic head primary and 19 had pancreatic body tumors, with 19 patients undergoing margin accentuation with IRE and 35 undergoing in situ IRE. Forty-nine had pre-IRE chemotherapy alone or chemo-radiation therapy for a median duration of 5 months. Forty (73%) patients underwent post-IRE chemotherapy or chemo-radiation. The 90 day mortality in the IRE patients was one (2%). In a comparison of IRE-treated patients to those receiving standard therapy alone we have seen an improvement in local progression free survival (14 vs. 6 months, P=0.01), distant progression free survival (15 vs. 9 months, P=0.02), and overall survival (20 vs. 13 months, P=0.03).
Acknowledgements
None.
Footnote
Conflicts of Interest: The author is consultant for Angiodynamics and the sole person responsible for the creation and editing of this article.
Ethical Statement: The study was approved by the University of Louisville IRB. Written informed consent was obtained from the patient. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- Arnoletti JP, Frolov A, Eloubeidi M, et al. A phase I study evaluating the role of the anti-epidermal growth factor receptor (EGFR) antibody cetuximab as a radiosensitizer with chemoradiation for locally advanced pancreatic cancer. Cancer Chemother Pharmacol 2011;67:891-7. [PubMed]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [PubMed]
- Bilimoria KY, Bentrem DJ, Merkow RP, et al. Application of the pancreatic adenocarcinoma staging system to pancreatic neuroendocrine tumors. J Am Coll Surg 2007;205:558-63. [PubMed]
- Artinyan A, Soriano PA, Prendergast C, et al. The anatomic location of pancreatic cancer is a prognostic factor for survival. HPB (Oxford) 2008;10:371-6. [PubMed]
- Kwon D, McFarland K, Velanovich V, et al. Borderline and locally advanced pancreatic adenocarcinoma margin accentuation with intraoperative irreversible electroporation. Surgery 2014;156:910-20. [PubMed]
- Al-Sakere B, André F, Bernat C, et al. Tumor ablation with irreversible electroporation. PLoS One 2007;2:e1135. [PubMed]
- Edd JF, Horowitz L, Davalos RV, et al. In vivo results of a new focal tissue ablation technique: irreversible electroporation. IEEE Trans Biomed Eng 2006;53:1409-15. [PubMed]
- Davalos RV, Mir IL, Rubinsky B. Tissue ablation with irreversible electroporation. Ann Biomed Eng 2005;33:223-31. [PubMed]
- Davalos RV, Otten DM, Mir LM, et al. Electrical impedance tomography for imaging tissue electroporation. IEEE Trans Biomed Eng 2004;51:761-7. [PubMed]
- Martin RC. Irreversible electroporation of locally advanced pancreatic head adenocarcinoma. J Gastrointest Surg 2013;17:1850-6. [PubMed]
- Martin RC 2nd, McFarland K, Ellis S, et al. Irreversible electroporation therapy in the management of locally advanced pancreatic adenocarcinoma. J Am Coll Surg 2012;215:361-9. [PubMed]
- Martin RC 2nd, McFarland K, Ellis S, et al. Irreversible electroporation in locally advanced pancreatic cancer: potential improved overall survival. Ann Surg Oncol 2013;20 Suppl 3:S443-9. [PubMed]
- Callery MP, Chang KJ, Fishman EK, et al. Pretreatment assessment of resectable and borderline resectable pancreatic cancer: expert consensus statement. Ann Surg Oncol 2009;16:1727-33. [PubMed]
- Varadhachary GR, Tamm EP, Abbruzzese JL, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol 2006;13:1035-46. [PubMed]
- Martin RC 2nd. The use of IRE in the treatment of a locally advanced pancreatic cancer (stage 3) of a pancreatic body/neck tumor. Asvide 2015;2:045. Available online: http://www.asvide.com/articles/501
- Bower M, Sherwood L, Li Y, et al. Irreversible electroporation of the pancreas: definitive local therapy without systemic effects. J Surg Oncol 2011;104:22-8. [PubMed]
- Charpentier KP, Wolf F, Noble L, et al. Irreversible electroporation of the pancreas in swine: a pilot study. HPB (Oxford) 2010;12:348-51. [PubMed]
Cite this article as: Martin RC 2nd. Irreversible electroporation of stage 3 locally advanced pancreatic cancer: optimal technique and outcomes. J Vis Surg 2015;1:4.


