
Robotic assisted thoracoscopic middle lobe sleeve resection: a case report
Introduction
More than fifty years after the first bronchial sleeve resection by Sir Clement Price Thomas [1893–1973], a meta-analysis by Ferguson and Lehman demonstrated that sleeve resections—if technically feasible—were superior to pneumonectomies when looking at 5-year-survival, quality-adjusted life-years and cost-effectiveness, and this in patients with early-stage lung cancer with acceptable lung function (1,2). More recently, several reports have demonstrated feasibility of minimal invasive sleeve resections (3-5). Although bronchial sleeve resections are mostly performed to avoid a pneumonectomy, they can also be performed to avoid a bilobectomy or even lobectomy in case of segmental sleeve resections (6,7).
I present a case of a symptomatic typical carcinoid originating from the orifice of the middle lobe bronchus. A middle lobe resection was performed in combination with an anastomosis of the lower lobe bronchus to the intermediate bronchus. Due to the anatomical proximity of the segmental bronchial orifices of the lower lobe, this operation might not be feasible oncologically due to insufficient margin of resection. The bronchial anatomy and, furthermore, the spatial relation to the pulmonary artery, makes a middle lobe sleeve resection technically demanding and is another reason why it is rarely executed.
In this report, the technical issues concerning a minimal invasive sleeve middle lobectomy and the potential advantage of using a robotic platform are discussed. The following case is presented in accordance with the CARE reporting checklist (available at https://jovs.amegroups.com/article/view/10.21037/jovs-20-50/rc).
Case presentation
A sportsman in his mid-twenties presented to his general physician with coughing and purulent sputa. A chest X-ray revealed a middle lobe pneumonia which was treated with antibiotics with good result. Two weeks later he had one episode of haemoptysis with a cloth in the shape of a casting of an airway branch. A chest CT revealed fibrotic atelectasis of the middle lobe and a complete obstruction of the middle lobe bronchus by soft tissue. In retrospect, a chest X-ray performed during a short hospitalization for a commotio cerebri three years earlier, already showed atelectasis of the middle lobe.
A bronchoscopy revealed a smooth mass protruding from the orifice of the middle lobe bronchus, pathology of the biopsy revealed a typical carcinoid.
A somatostatin receptor PET/CT scan (68Ga-DOTATATE) showed a single lesion at the level of the middle lobe bronchus without other local or distant lesions. The patient had a normal spirometry and diffusion capacity. He was counselled and agreed with primary resection of the lesion.
Intervention
Middle lobe sleeve resections can be performed through different approaches e.g. thoracotomy, multiport VATS or uniportal VATS (8-10). In Video 1, the author demonstrates the feasibility by robotic assisted thoracoscopic surgery (RATS) as it has been described by Durand et al. (11).
Preparation & installation
The procedure was performed with an Xi® system (Intuitive Surgical®) and 30° 8 mm camera. Bronchoscopic visualization of the tumour is advised both prior to the start of surgery and at the moment of bronchial incision.
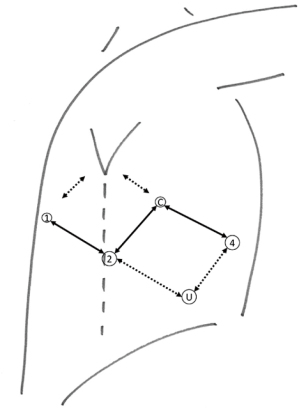
After the start of the general anaesthesia and the placement of a double lumen tube, the patient was installed in lateral decubitus. After draping and time-out, an utility incision was made for a 12 mm trocar at the 7th intercostal space at 5cm from the costal margin (Figure 1). CO2 insufflation was started at a pressure of 6 mmHg. Four additional trocars were placed in an ‘Ursa Major’ configuration and the robotic cart was docked. This configuration has a caudal position of the ports of the working arms to the left and right of the camera and allows more degree of freedom for the Intuitive staplers. In case these are not used, a more typical port placement on a single line can be used. In the most posterior port, we used a tip-up fenestrated grasper for lung retraction; in the port to the left of the camera a Prograsp® forceps and to the right a Maryland bipolar forceps, switching to scissors and needle driver when necessary. The ports flanking the camera, were changed to 12 mm ports to use Intuitive staplers. Two handmade rolled gauzes were inserted for lung retraction. The assistant handled a 5 mm suction device to aspirate smoke and blood and removed lymph nodes within finger gloves by the 12 mm utility port.
Resection
We like to perform the mediastinal lymph node dissection first: transection of the pulmonary ligament, identification of the lower lobe vein, opening of the posterior pleural reflexion, lymphadenectomy of the subcarinal region, dissection of the posterior side of the upper lobe bronchus and intermediate bronchus, visualizing the secondary carina, opening of the anterior pleural reflexion, identification of the superior vein, lymphadenectomy below the azygos vein and of the paratracheal region.
The fissure between middle lobe and lower lobe is usually well developed and allows access to the pulmonary artery at the level of the most caudal of the two middle lobe arteries (A5). If not, the fissure can be opened by an anterior tunnelling technique between the artery and parenchyma as previously described (12). After transection of the middle lobe artery and vein, the middle lobe bronchus is freed as much as possible.
One of the technical hurdles of a sleeve middle lobectomy is the overlying lower lobe pulmonary artery, hampering the access to the distal intermediate bronchus and the orifices of lower lobe bronchial segments. Second, during the bronchial section and anastomosis, one has to be careful not to comprise the segments of the lower lobe as the level of the distal margin of the sleeve is located at the orifices of the lower lobe segments.
To enhance the exposure, a sling can be pulled around the lower lobe artery. The intermediate bronchus should be freed from the adjacent lower lobe vein and pulmonary artery. Even then, transection of the lower lobe bronchus (basal segments and S6 together) can be difficult. While the rule is to keep the tumour attached to the resected lobe, alternatively and only in small tumours, the bronchus can be transected distally to the tumour to obtain a good vision. Next, the bronchus can be cut longitudinally opposite to the tumour implantation towards centrally. The lower lobe and intermediate bronchus must be cut well away from the tumour while obtaining a direct view on the tumour. In case that the tumour is too big to allow transection the distal part of the middle lobe bronchus first, endobronchial visualization should guide the transection of intermediate bronchus and lower lobe bronchus. At all time, one has to be careful not to cut too deep into the lower lobe segmental bronchi. In the case shown in the video, the first cut in the bronchus is made with a view from anterior on the middle lobe bronchus, while pulling the pulmonary artery posteriorly. The further resection, including obtaining section margins, and further sleeve anastomosis was done from a posterior view while pulling the artery anteriorly. To deal with this specific technicality and exposure, we trained this procedure in the cadaver lab proctored by Dr. Marion Durand and found this to be very helpful (Figure 2). Note that during the handling of the bronchus the light of the bronchoscope can easily be seen transbronchially with the infrared mode (Firefly®, Intuitive Surgical®) which gives extra guidance.
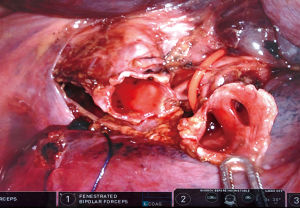
The anastomosis was made with the use of V-Loc™ 3/0 180 (Medtronic). Barbed wire is helpful as the tissue stays approximated during the anastomosis. The anastomosis can be made starting in the 4 o’clock (anterior) position. A continuous suture is performed over the mediastinal part towards posterior in clockwise direction (11). A new suture is placed at the level of the first suture and a continuous suture is performed, knotting the two ends at the lateral level of the anastomosis. The airtightness should be checked by insufflation under water.
Peri-operative bronchoscopy allows a quality check of the anastomosis. A single misplaced stitch can result in narrowing of the orifices of the lower lobe segments.
Pathology & follow-up
Final pathology confirmed a typical carcinoid of 1.4 cm. The bronchial margins of the intermediate bronchus and lower lobe bronchus were free of tumour. The mediastinal, hilar and interlobar lymph node stations were all negative. Bronchoscopy at day 2 showed an inflamed but patent anastomosis visualizing both S6 and S7–10 segmental orifices. The thoracic drain was removed at day 4 when fluid output was less than 450 cc. Hospital stay was 6 days. Around day 60 the patient consulted because of persisting coughing, this was treated conservatively and absent at day 90. At one year, the patient was symptom free. Lung function test was normal. A CT-scan showed the usual postoperative changes with a patent bronchus to the lower lobe. Further follow-up up to ten years after surgery is planned as recurrence of typical carcinoid presents late. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient.
Discussion
The author presented the technical steps of a sleeve middle lobectomy on a robotic platform. While the feasibility of a VATS sleeve middle lobectomy has been beautifully demonstrated by Dr. Gossot and Dr. Gonzalez Rivas, and the author has experience with uniportal and multiportal VATS sleeve resections, I think that—certainly for this procedure—the robotic platform can bring added value (8,10). The lateral 30° camera position allows both anterior and posterior view, anterior for a classic middle lobe resection, followed by a posterior view for the sleeve anastomosis. The third instrument port allows retraction of the overlying pulmonary artery that obstructs the view to the distal intermediate bronchus and lower lobe segments. Suturing at the level of the orifices of the segmental bronchi is technically demanding, but facilitated through the robotic platform. However, even with the availability of a robot, the surgeon needs to be experienced to achieve a good result.
To avoid losing tension and locking while stitching, short barbed wires can be used (15 cm V-Loc® 3/0 or 4/0). Avoiding the necessity of a first knot is an additional feature. Results of widespread use of this type of wire in bronchial anastomosis should be published. Durand et al. and Cosgun et al. found no issues in their robotic sleeve procedures with this type of wire, Nakagawa et al. had one anastomotic stenosis out of a total of four cases, not needing any re-intervention (11,13,14). Another advantage of the robotic platform is the ease of the lymphadenectomy. Debate on the quality of nodal (mediastinal) clearance, comparing open, robotic and VATS approaches, is ongoing, with RATS showing equal or better results than VATS (15-17).
Due to the smaller size of the middle lobe, one might argue that a sleeve middle lobe resection should mainly be reserved for patients with a low lung function, where a lower bilobectomy should be avoided (9). However, the risk of morbidity and mortality after bilobectomy is typically quoted to be somewhere between a lobectomy and pneumonectomy (18). More specifically, while an upper bilobectomy has a similar risk not much higher than an upper lobectomy, a lower bilobectomy had in the study of Thomas et al. mortality risk comparable to a left pneumonectomy, but lower than a right pneumonectomy (18). Data demonstrating lower morbidity or mortality risks with sleeve resections of the middle lobe are missing, but a sleeve middle lobectomy might be preferable over a lower bilobectomy, if oncologically and technically feasible, even in patients with a reasonable lung function.
Typical indications are small intrabronchial lesions at the orifice of the middle lobe bronchus without involvement of the lower lobe segmental bronchi.
Absolute contraindication for a sleeve middle lobectomy is a situation where avoiding a bilobectomy would result in an incomplete resection. Especially when positive nodes or an extra-bronchial involvement of the tumour at the level of the tertiary carina are present, care has to be taken to achieve a complete resection, preferably by a bilobectomy.
In conclusion, a sleeve middle lobectomy is feasible minimally invasively. Performing a minimally invasive sleeve middle lobectomy avoids a thoracotomy and lower bilobectomy. The main advantage of the robotic platform is, first, the possibility of obtaining a good exposure despite the overlying pulmonary artery of the lower lobe. An anterior view can be obtained for the middle lobe resection and a posterior view for the anastomosis of the intermediate bronchus with lower lobe bronchus. Second, the robotic platform helps exact angulation of the needle and careful stitching close to the orifices of the segments.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Jean-Marc Baste) for the series “Robotic Assisted Thoracic Surgery: Advanced Procedures in Lung and Mediastinum: From Post-induction TTT (immunotherapy) to Sleeve Resection, Complex Segmentectomies and Extended Thymectomy for Myasthenia Gravis” published in Journal of Visualized Surgery. The article has undergone external peer review.
Reporting Checklist: The author has completed the CARE reporting checklist. Available at https://jovs.amegroups.com/article/view/10.21037/jovs-20-50/rc
Peer Review File: Available at https://jovs.amegroups.com/article/view/10.21037/jovs-20-50/prf
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.com/article/view/10.21037/jovs-20-50/coif). The series “Robotic Assisted Thoracic Surgery: Advanced Procedures in Lung and Mediastinum: From Post-induction TTT (immunotherapy) to Sleeve Resection, Complex Segmentectomies and Extended Thymectomy for Myasthenia Gravis” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images/video. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Thomas CP. Conservative resection of the bronchial tree. J R Coll Surg Edinb 1956;1:169-86. [PubMed]
- Ferguson MK, Lehman AG. Sleeve lobectomy or pneumonectomy: optimal management strategy using decision analysis techniques. Ann Thorac Surg 2003;76:1782-8. [Crossref] [PubMed]
- Caso R, Watson TJ, Khaitan PG, et al. Outcomes of minimally invasive sleeve resection. J Thorac Dis 2018;10:6653-9. [Crossref] [PubMed]
- Gao HJ, Jiang ZH, Gong L, et al. Video-Assisted Vs Thoracotomy Sleeve Lobectomy for Lung Cancer: A Propensity Matched Analysis. Ann Thorac Surg 2019;108:1072-9. [Crossref] [PubMed]
- Jiao W, Zhao Y, Qiu T, et al. Robotic Bronchial Sleeve Lobectomy for Central Lung Tumors: Technique and Outcome. Ann Thorac Surg 2019;108:211-8. [Crossref] [PubMed]
- Tsutani Y, Okada M. Bronchoplasties at the Segmental Level. Thorac Surg Clin 2018;28:299-304. [Crossref] [PubMed]
- Nagashima T, Inui K, Kanno K, et al. Thoracoscopic right S6 sleeve segmentectomy for squamous-cell carcinoma arising from the B6 central bronchus. J Thorac Dis 2018;10:1077-80. [Crossref] [PubMed]
- Gossot D, Grigoroiu M, Brian E. Thoracoscopic middle lobectomy with sleeve resection for bulky carcinoid tumor. Epublication WebSurg.com, Apr 2014;14(04). [cited 2020 Jul 4]. Streaming video: 09:20 min. Available online: https://websurg.com/tw/doi/vd01en4215/
- Antonoff MB, Meyers BF. Right Middle Lobe Sleeve Resection - Master Techniques in Surgery: Thoracic Surgery: Lung Resections, Bronchoplasty, 1st Ed. Lippincott Williams & Wilkins (LWW) 2014.
- Gonzalez-Rivas D. Uniportal VATS middle sleeve lobectomy; 2019 Mar 19 [cited 2020 Jul 4]. Streaming video: 17:12 min. Available online: https://www.youtube.com/watch?v=L5JtJpa-fWA. n.d.
- Durand M. Robotic bronchial sleeve resections: technical details and early results. Mini-invasive Surg 2019;2019:1-9. [Crossref]
- Decaluwe H. Video-assisted thoracic surgery tunnel technique: an alternative fissureless approach for anatomical lung resections. Video-assist Thorac Surg 2017;2:45. [Crossref]
- Cosgun T, Kaba E, Ayalp K, et al. Bronchial sleeve anastomosis and primary closures with the da Vinci system: an advanced minimally invasive technique. Video-assist Thorac Surg 2017;2:49. [Crossref]
- Nakagawa T, Chiba N, Ueda Y, et al. Clinical experience of sleeve lobectomy with bronchoplasty using a continuous absorbable barbed suture. Gen Thorac Cardiovasc Surg 2015;63:640-3. [Crossref] [PubMed]
- Kneuertz PJ, Cheufou DH, D’Souza DM, et al. Propensity-score adjusted comparison of pathologic nodal upstaging by robotic, video-assisted thoracoscopic, and open lobectomy for non-small cell lung cancer. J Thorac Cardiovasc Surg 2019;158:1457-66.e2. [Crossref] [PubMed]
- Yang HX, Woo KM, Sima CS, et al. Long-term Survival Based on the Surgical Approach to Lobectomy For Clinical Stage I Non-small Cell Lung Cancer: Comparison of Robotic, Video-assisted Thoracic Surgery, and Thoracotomy Lobectomy. Ann Surg 2017;265:431-7. [Crossref] [PubMed]
- Hennon MW, DeGraaff LH, Groman A, et al. The association of nodal upstaging with surgical approach and its impact on long-term survival after resection of non-small-cell lung cancer. Eur J Cardiothorac Surg 2020;57:888-95. [Crossref] [PubMed]
- Thomas PA, Falcoz PE, Bernard A, et al. Bilobectomy for lung cancer: contemporary national early morbidity and mortality outcomes. Eur J Cardiothorac Surg 2016;49:e38-43. [Crossref] [PubMed]
Cite this article as: Decaluwé H. Robotic assisted thoracoscopic middle lobe sleeve resection: a case report. J Vis Surg 2022;8:7.


