
Reconstruction surgery case report: ex vivo surgery and auto lung transplantation for pulmonary artery sarcoma
Introduction
Pulmonary artery (PA) sarcoma has been reported as an extremely rare tumor with a very poor prognosis. Surgical intervention, including pulmonary endarterectomy and pneumonectomy, is considered the mainstay of treatment to relieve symptoms and offer a chance for a long-term survival (1-5). However, the optimal surgical approach must be determined individually, based on the tumor location and the patients’ clinical condition.
Lung autotransplantation has been successfully employed for various types of lung disease in order to spare the pulmonary parenchyma, preserving the lung function (6-15). We herein report our experience performing lung autotransplantation with ex vivo bench surgery for the treatment of rare intimal sarcoma of the PA in accordance with the CAse REport (CARE) guideline (16).
Case presentation
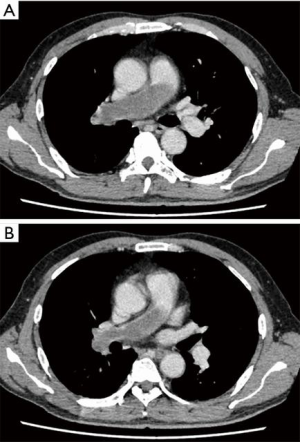
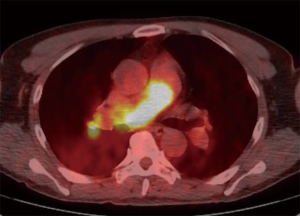
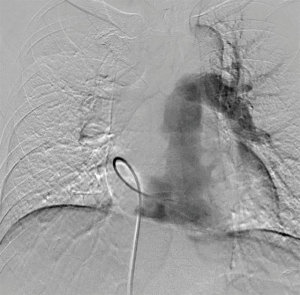
A 58-year-old man was referred to our hospital with a fever and dyspnea on exertion. Enhanced computed tomography showed low-attenuation filling defects occupying the entire luminal diameter of the right main PA, peripherally extending into the right interlobar PA (Figure 1). Positron emission tomography demonstrated an intense uptake of florine-18 fluorodeoxyglucose in the intraluminal defects, with a maximum standardized uptake value of 15.2 (Figure 2). A pulmonary angiogram showed severe stenosis of the right main PA (Figure 3). Right heart catheterization revealed a severely elevated systolic right ventricular pressure of 76 mmHg. Pulmonary function tests showed a mild restrictive ventilatory disorder with vital capacity of 3.05 L (79.6% of predicted value) and forced expiratory volume in 1 second of 2.20 L (70.1% of predicted value). The patient’s condition rapidly deteriorated and he became bed-bound. Based on these findings, PA sarcoma was clinically suspected, and the initial surgical strategy consisted of pulmonary endarterectomy and right pneumonectomy, followed by reimplantation of the right lower lobe in order to preserve the post-operative pulmonary function. All procedures performed in this study were in accordance with the Helsinki Declaration (as revised in 2013). Informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
The surgical procedure is demonstrated in Video 1. Through median sternotomy combined with a fourth intercostal right thoracotomy, cardiopulmonary bypass was established through the ascending aortic and bicaval cannulation. The aorta was cross-clamped, and cardiac arrest was commenced. The longitudinal incision of the PA trunk and right main PA was performed in order to remove the centrally located tumor, originating in the posterior wall of the PA immediately above the pulmonary valve. After the right main PA had been reconstructed, the heartbeat was resumed. The cardiac arrest time was 66 minutes.
Right pneumonectomy was then performed in order to remove the residual tumor within the peripheral PA. A subsequent ex vivo assessment of the explanted right lung revealed that the right lower PA was intraluminally tumor-free, so the right lower lobe could be reimplanted. The right upper and middle lobes were resected ex vivo, and antegrade and retrograde flushing of the remaining healthy lower lobe was then performed with approximately 2,000 mL of ET Kyoto solution (Otsuka Pharmaceutical Factory, Tokushima, Japan) at 4 °C on a back table, based on our established protocol for living donor lobar lung transplantation (18,19). We created a homologous pulmonary arterial graft conduit using a chest tube as a mould which was used for elongation of the graft PA. The implantation was performed as follows: the graft bronchus was anastomosed to the right main bronchus with running 4-0 PDS sutures for the membranous portion and interrupted 4-0 PDS sutures for the cartilaginous portion. The bronchial anastomotic site was then wrapped with a pedicled pericardial fat pad. The reconstructed graft PA was directly anastomosed to the right main PA with running 6-0 Prolene sutures. The pulmonary vein of the graft was anastomosed to the remaining upper pulmonary venous stump with running 6-0 Prolene sutures. The total ischemic time of the graft was 118 minutes, and no ischemia-reperfusion injury was noted after transplantation. The patient was successfully weaned from the cardiopulmonary support immediately after reperfusion. The cardiopulmonary bypass time was 253 minutes.
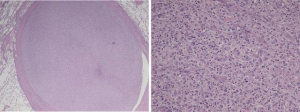
The histopathological features of the tumor were those of a pleomorphic malignancy comprising spindled and epithelioid cells admixed with inflammatory cells (Figure 4). Areas of necrosis and mitoses were sporadically observed. There was no local invasion of the tumor cells outside the vessel wall. These findings were consistent with a diagnosis of PA intimal sarcoma.
His early post-transplant course was uneventful: The patient was weaned from the mechanical ventilatory support on postoperative day (POD) 2 and discharged from the intensive-care unit on POD 6. He returned to his normal life one month after surgery. He died of tumor recurrence at the distal left PA and liver and bone metastasis 14 months after surgery.
Discussion
The lung autotransplant procedure has been previously reported as a useful technique for the treatment of various types of lung disease, including centrally located lung cancer, postpneumonectomy-like syndrome, locally advanced lung cancer, and bronchopleural fistula after right upper bronchial sleeve lobectomy (6-15). We herein reported the utility of ex vivo bench surgery and autotransplantation to completely resect the intimal sarcoma of the PA, thereby preserving the post-operative pulmonary function.
In the present case, the benefits of ex vivo surgery were considered to be as follows: (I) extended resection of tumor could be safely performed on a back table with a favorable visual field and relatively little tumor manipulation; (II) antegrade and retrograde flushes of the lung graft with cold organ preservation solution helped avoid thrombus formation in the graft and ameliorate ischemia-reperfusion-induced lung injury after reimplantation; and (III) cold lung preservation provided enough time to pathologically confirm the tumor-free surgical margins.
PA sarcoma has been reported as an extremely rare tumor with a very poor prognosis. Although only surgical resection, commonly including pneumonectomy or pulmonary endarterectomy, is considered the mainstay of treatment to prolong the survival for patients with this orphan disease, the reported median survival time was 1.5 months without surgery and 10 to 18 months even with surgical resection (1-5). In this case, the patient died of recurrence of PA sarcoma 14 months postoperatively. However, no major complications were observed in the early postoperative course, and he was able to return to his normal life one month after surgery, although he was completely bed-bound before surgery.
Conclusions
We reported a case of PA intimal sarcoma that was completely resected with pulmonary endarterectomy and right pneumonectomy, followed by ex vivo surgery and lung autotransplantation. This complicated procedure was found to be technically feasible without any major post-operative complications and may be an optimal surgical approach for select patients with PA sarcoma.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Meinoshin Okumura) for the series “Dedicated to the 36th Annual Conference of Japanese Association for Chest Surgery” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jovs.amegroups.com/article/view/10.21037/jovs.2019.10.06/coif). The series “Dedicated to the 36th Annual Conference of Japanese Association for Chest Surgery” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the Helsinki Declaration (as revised in 2013). Informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Krüger I, Borowski A, Horst M, et al. Symptoms, Diagnosis, and Therapy of Primary Sarcomas of the Pulmonary Artery. Thorac Cardiovasc Surg 1990;38:91-5. [Crossref] [PubMed]
- Mussot S, Ghigna MR, Mercier O, et al. Retrospective institutional study of 31 patients treated for pulmonary artery sarcoma. Eur J Cardiothorac Surg 2013;43:787-93. [Crossref] [PubMed]
- Yin K, Zhang Z, Luo R, et al. Clinical features and surgical outcomes of pulmonary artery sarcoma. J Thorac Cardiovasc Surg 2018;155:1109-1115.e1. [Crossref] [PubMed]
- Grazioli V, Vistarini N, Morsolini M, et al. Surgical treatment of primary pulmonary artery sarcoma. J Thorac Cardiovasc Surg 2014;148:113-8. [Crossref] [PubMed]
- Deng L, Zhu J, Xu J, et al. Clinical presentation and surgical treatment of primary pulmonary artery sarcoma. Interact Cardiovasc Thorac Surg 2018;26:243-7. [Crossref] [PubMed]
- Hamaji M, Chen-Yoshikawa TF, Date H. Completion pneumonectomy and auto-transplantation for bronchopleural fistula. J Thorac Cardiovasc Surg 2019;158:e121-3. [Crossref] [PubMed]
- Tanaka S, Sugimoto S, Soh J, et al. Long-term outcomes of pneumonectomy, back-table lung preservation, double-sleeve resection and reimplantation for advanced central lung cancer: the Oto procedure. Eur J Cardiothorac Surg 2019;56:213-4. [Crossref] [PubMed]
- Watanabe Y, Sato M, Nakamura Y, et al. Right Lower Lobe Autotransplantation for Locally Advanced Central Lung Cancer. Ann Thorac Surg 2015;99:323-6. [Crossref] [PubMed]
- Yamashita T, Hamaji M, Nakanobo R, et al. Ex Vivo Sleeve Lobectomy and Autotransplantation After Chemoradiation. Ann Thorac Surg 2019;107:e341-e343. [Crossref] [PubMed]
- Chen F, Takahagi A, Sakamoto K, et al. Lung autotransplantation technique for postpneumonectomy-like syndrome. J Thorac Cardiovasc Surg 2015;150:e45-7. [Crossref] [PubMed]
- Oto T, Kiura K, Toyooka S, et al. Basal segmental auto-transplantation after pneumonectomy for advanced central lung cancer. Eur J Cardiothorac Surg 2012;42:579-81. [Crossref] [PubMed]
- Tryfon S, Zarogoulidis P, Tsavlis D, et al. Ex situ reimplantation technique, in central lung tumors. Ann Transl Med 2015;3:178. [PubMed]
- Jiang F, Xu L, Yuan F, et al. Lung Autotransplantation Technique in the Treatment for Central Lung Cancer of Upper Lobe. J Thorac Oncol 2008;3:609-11. [Crossref] [PubMed]
- Reardon MJ, Walkes JC, Rice DC. Autotransplantation for central non-small-cell lung cancer in a patient with poor pulmonary function. Tex Heart Inst J 2004;31:360-2. [PubMed]
- Karube Y, Chida M, Nishihira M, et al. Back-table procedure and auto-lung transplantation for locally advanced lung cancer: a case report. J Cardiothorac Surg 2016;11:3. [Crossref] [PubMed]
- Riley DS, Barber MS, Kienle GS, et al. CARE guidelines for case reports: explanation and elaboration document. J Clin Epidemiol 2017;89:218-35. [Crossref] [PubMed]
- Nakajima D, Miyahara S, Date H. Lung autotransplant technique with ex vivo surgery for pulmonary artery sarcoma. Asvide 2019;6:322. Available online: http://www.asvide.com/watch/33007
- Date H, Aoe M, Nagahiro I, et al. Living-donor lobar lung transplantation for various lung diseases. J Thorac Cardiovasc Surg 2003;126:476-81. [Crossref] [PubMed]
- Date H, Sato M, Aoyama A, et al. Living-donor lobar lung transplantation provides similar survival to cadaveric lung transplantation even for very ill patients†. Eur J Cardiothorac Surg 2015;47:967-72; discussion 972-3. [Crossref] [PubMed]
Cite this article as: Nakajima D, Miyahara S, Date H. Reconstruction surgery case report: ex vivo surgery and auto lung transplantation for pulmonary artery sarcoma. J Vis Surg 2020;6:2.


