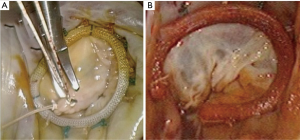
The principles of minimally invasive atrioventricular valve repair surgery utilizing endoaortic balloon occlusion technology: how to start and sustain a safe and effective program
Introduction
Transcatheter- (TC) and minimally invasive atrioventricular valve surgical (MIAS) technology are rapidly evolving and are paralleled by increasing patient expectations (1), quality control (2), clinical governance (3) and patient risk profiles (4). In an era of decreasing surgical volume, training opportunities, operative exposure and constrains in healthcare cost, upcoming surgeons are required to master challenging MIAS learning curves while maintaining acceptable clinical outcomes (5,6). It is now well recognised that both current- and future cardiac surgical practices will be expected to be proficient in MIAS and TC device implantation (7,8), but the implementation and maintenance or such programs require systematic logistical and infrastructure planning. Port Access™ atrioventricular valve surgery (PAS) utilizes videoscopic- or robotic vision, modified instruments, transoesophageal echocardiographic- (TEE) or fluoroscopic guided peripheral cardiopulmonary bypass (CPB) and endo-aortic balloon technology or external aortic clamping techniques to facilitate primary- (9,10) and redo- (11,12) MIAS repair- and replacement procedures. Between February 1st 1998 and May 31st 2019, a total of 3,180 patients underwent PAS procedures at our institution for isolated atrioventricular valve (AVV) pathology. This manuscript provides an overview of the historic evolution of PAS, contemporary PAS technology, PAS infrastructure planning and the operative principles of PAS to assist new centres to establish and maintain safe- and effective PAS programs.
Historical evolution of port access™ surgery (PAS)
The concept of PAS was originally developed in 1994 (Heartport Inc., Redwood City, CA, USA) with the intention of using any combination of central- or peripheral CPB, endo-aortic balloon occlusion-, retrograde cardioplegia- and pulmonary artery venting catheter devices to facilitate minimally invasive cardiac surgical procedures. Animal studies at Stanford- and New York University (13) demonstrated PAS feasibility and safety with subsequent United States Food and Drug Administration approval in 1996 Ame (14-17). More than 18,000 minimally invasive cardiac procedures, which included coronary artery bypass grafting, were performed using components of PAS technology between 1996 and 2000 and its ergonomic advantage of allowing MIAS through small working ports without additional external instrumentation (18-20), especially in total endoscopic- (21-23) and robotic surgery (24,25), became well recognised. Edwards Lifesciences (Irvine, California, USA) subsequently took ownership of PAS and recently reported a global use of PAS technology in 12,689 patients between 2014 and 2017.
Current port access™ technology
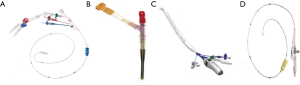
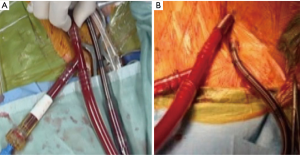
IntraClude™ (Edwards Lifesciences, Irvine, California, USA) is a composite endo-aortic balloon occlusion device (10.5 Fr, 100 cm length) that facilitates antegrade cardioplegia delivery, aortic root venting and pressure monitoring for ascending aorta sizes ranging 20–40 mm (Figure 1A). It is advanced to the sino-tubular junction under TEE- or fluoroscopic guidance over a 200 cm 0.0038 J-tip guidewire through the side arm of the EndoReturn™ femoral arterial cannula (21–23 Fr, Edwards Lifesciences, Irvine, California, USA) (Figure 1B). The QuickDraw™ femoral venous cannula (22–25 Fr, Edwards Lifesciences, Irvine, California, USA) is inserted under TEE or fluoroscopic guidance into the right atrium and is compatible with percutaneous approaches. The ProPlege™ peripheral retrograde cardioplegia device (9 Fr, 59 cm, Edwards Lifesciences, Irvine, California, USA), is a triple lumen device that is inserted through an internal jugular vein sheath into the coronary sinus under TEE guidance as an adjunct to endo-aortic balloon occlusion for additional retrograde cardioplegia delivery (Figure 1C). The EndoVent™ pulmonary catheter (8.3 Fr, Edwards Lifesciences, Irvine, California, USA) is inserted though the internal jugular- or subclavian vein as an additional endo-pulmonary artery venting device (Figure 1D). Optisite™ (17 Fr, Edwards Lifesciences, Irvine, California, USA) is a peripheral cannula that can be utilized for additional arterial cannulation in cases of high CPB flow pressures. Special atrial retractors facilitate intracardiac access to perform robotic- or endoscopic AVV repair and replacement procedures.
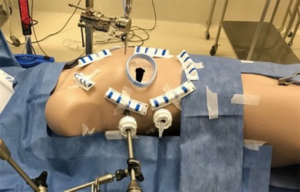
Infrastructure planning
PAS health care economics
Evidence of proven efficacy, safety, feasibility and cost-effectiveness are required to obtain institutional support as a first step in initiating a PAS program. Atluri (26) and Santana (27) independently suggested that the greater operative cost associated with MIAS is offset by reduced costs of cardiac imaging, laboratory tests, lower use of blood products, fewer perioperative infections, faster recovery, shorter hospital length of stay, fewer requirements for rehabilitation and lower readmission rates. Equipment acquisition- and operative theatre upgrades account for the majority of the initial institutional capital investment and the disposable costs can subsequently be offset against the cost savings mentioned.
PAS and hybrid operative theatre design
Modern MIAS- and hybrid cardiovascular- and thoracic operating rooms are designed to facilitate TC and MIAS procedures in conjunction with efficient workflow, safety, access, lights, imaging modalities and theatre hygiene. Building a “state of the art” hybrid operating room (Figure 2) is a considerable economic investment for every institution, but various reports from the United States and China (28), suggest that case load potentially triple based upon the hybrid theatre setup, with a complete return on investment within 2 years. For PAS, the basic operative room layout must be able to accommodate a cardiac anaesthetic- and TEE machine, an endoscopic camera- and CO2 delivery stack, a CPB machine, various synchronised screens for neuro-cardio-respiratory-, TEE- and 2D and 3D endoscopic image projection and adequate ergonomics that can accommodate 2 anaesthetists, 2 perfusionists, 2 surgeons, a theatre nurse and a support nurse. It is imperative that all routine cardiovascular equipment, guidewires, grafts, stents and sutures are readily available if required.

Teamwork, communication, ownership and leadership
To promote trust, communication, effective teamwork, training and education, established PAS centres advocate a patient-centred-, multidisciplinary cardiac operative team that include an experienced anaesthesiologist (29), perfusionist (30), a skilled cardiac surgeon, surgical assistant, an experienced operative nurse and cardiac nursing support. It is suggested that a dedicated theatre team visit established PAS centres for training and mentorship and that a constant team initiate the PAS program for at least 20 cases (31). Frequent constructive postoperative team debriefing sessions that focus on continuous improvement strategies are invaluable and reinforces ownership of each team member under the surgical leadership. Intraoperative communication is essential and each team member`s opinion and concerns should be respected and addressed throughout any PAS procedure. For continuation of postoperative care, the expanded team members include skilled intensive care-, ward- and outpatient nurses, physiotherapists, other allied health care professionals, the patient family and referring physicians.
Patient selection
In an era where surgical volume is progressively decreasing, emerging PAS programs should practice extreme caution in the initial patient- and valve pathology selection. Even though PAS is applied as a routine in experienced centres without exclusion criteria (32-37), it is suggested that emerging programs should preferably not offer PAS to patients with high risk clinical-, anatomical- and echocardiographic characteristics, which are outlined in Table 1. In addition to routine cardiac surgical preoperative investigations, evaluation of the aorta-iliac-femoral arterial-axis by contrasted computerised tomography, magnetic resonance imaging or an additional peripheral contrast injection during coronary angiography, is mandatory. In 511 consecutive patients that underwent PAS in an experience centre over a 5-year period, lung adhesions (n=5, 1.0%) and peripheral cannulation complications (n=4, 0.8%) required sternotomy conversion despite detailed preoperative evaluations (38).
Table 1
| Patient characteristics |
| Potential difficult access |
| Morbid obesity |
| Thoracic wall deformities |
| Previous right thoracotomy |
| Previous right thoracic radiation or trauma |
| Contraindications to- or unsuccessful right lung isolation |
| Previous right ilio-femoral peripheral vascular interventions |
| High surgical risk |
| Elderly and high frailty index |
| Previous cardiac surgery |
| Urgent/emergency status |
| Multi-organ dysfunction |
| Poor respiratory function |
| Other co-morbidities risking adverse perioperative outcomes |
| Vascular disease |
| Aorta-iliac-femoral artery-axis calcification, atheroma or aneurysms |
| Common femoral artery diameter smaller than 8 mm |
| Ascending aorta ectasia, dilatation or aneurysm larger than 40 mm |
| Sinu-tubular junction or aortic root dilatation more than 40 mm |
| Echocardiographic characteristics |
| Complex valve pathology for repair or replacement |
| Barlow`s morphology |
| Infective endocarditis |
| Severe posterior annular calcification |
| Aortic valve regurgitation |
| Advanced cardiomyopathy |
| Severe pulmonary hypertension |
Procedural overview
Patient positioning and port incisions
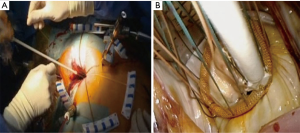
The patient is positioned supine with the right arm flexed and external defibrillation pads applied. An inflatable cushion is used to elevate the right hemithorax following routine cardiac anaesthesia that includes the insertion of a double lumen endotracheal tube, right internal jugular central venous- and right radial artery catheters, urinary catheter, rectal temperature monitoring and TEE probe. It is advised to utilise a right internal jugular venous cannula (16–18 Fr, Optisite™, Edwards Lifesciences, Irvine, California, USA) as additional venous drainage during the learning period. Various experienced centres however, rely only on single femoral venous cannulation augmented by vacuum assisted drainage. The use of bilateral radial artery catheter monitoring may be used during the early experience to ensure continuous hemodynamic monitoring in case of aortic dissection or endo-balloon dislodgement that may occlude the innominate artery. A 4-6 cm, non-rib spreading working port with a soft tissue retractor (SurgiSleeve™, 2.5–6 centimeters, Covidien, Massachusetts, USA) is established over the 4th intercostal, anterior axillary space, halfway between the clavicle and inferior xyphoid-costal border. The diaphragmatic dome should be palpable. A 7-mm port is inserted 2 to 3 intercostal spaces inferior to the working port for continuous CO2 insufflation and left atrial vent line placement. A 5-mm endoscopic port- and a 5-mm left atrial retractor shaft are inserted latero-posteriorly- and parasternal-medially to the working port in the 4th intercostal space respectively (Figure 3A). The retractor-shaft is externally anchored to a Bookwalther™ retractor (Symmetry Surgical, Tennessee, USA) and a steel wire introduced through a needle in 2nd intercostal space, mid-clavicular line to facilitate valve exposure by retracting annuloplasty sutures anterolaterally. Unobstructed visual- and working access are ascertained by resecting excessive pericardial fat (Figure 3B) and retracting the diaphragmatic dome infero-laterally with exteriorized traction sutures. The additional use of retrograde coronary sinus cannulation for cardioplegia delivery (ProPlege™) and pulmonary artery venting (EndoVent™) can be considered, but is not generally utilized during the initial learning curve (Figure 3C).
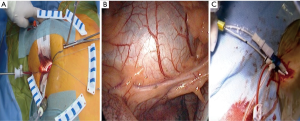
Peripheral vascular cannulation and IntraClude™ positioning
A 4-cm right groin incision provides access to the right common femoral artery and vein during which care is taken to avoid the medial lymphatic rich regions. Following systemic heparinization and confirmation of an activated clotting time more than 400 seconds, the femoral vein is punctured first by using the Seldinger technique. A radio-opaque guidewire is inserted into the right atrium under TEE or fluoroscopic guidance, after which the Quickdraw™ venous cannula is advanced over the guidewire and secured with the cannula tip adjacent to the intraatrial septum. Vacuum assist venous drainage is a necessary adjunct for femoral venous cannulation. The common femoral artery is similarly punctured above the deep branch bifurcation followed by the TEE guided insertion of a guidewire into the descending aorta. The artery is subsequent dilated and an appropriately sized EndoReturn™ cannula inserted, de-aired, secured and observed for pulsatile waveforms. The use of peripheral limb saturation monitoring is suggested to monitor for leg ischemia during peripheral CPB, but the routine use of distal perfusion strategies, which include additional cannulation, is controversial and not generally advocated. The Intraclude™ catheter device is inserted through the Endoreturn™ side-arm, de-aired and carefully advanced over its guidewire across the descending aorta, the aortic arch and into the ascending aorta under TEE guidance and is then locked into position (Figure 4A). Total percutaneous cannulation using vascular closure devices (39) can be performed as a favourable alternative (Figure 4B), but is not advocated if inexperienced. Peripheral vascular spasm may occur and can be identified by dampening of the arterial curve on the CPB machine, which then requires contralateral cannulation. This is achieved with the insertion of an Optisite™ cannula in the left common femoral artery, which is then connected to the Endoreturn™ tubing in a Y-configuration arterial inflow circuit. CPB- and systemic hypothermia to 32 degrees Celsius are carefully initiated. CPB pressures more than 300 mmHg require temporary flow cessation and contralateral cannulation as described. It is reported that insufficient CPB flow occur in 0.2%-, guidewire resistance in 0.6%- and cannulation related aortic dissection in 0.2% of PAS procedures, which emphasises the importance of meticulous preoperative aorta-iliac-femoral-axis evaluation, access planning and careful guidewire manipulation techniques (40). Central aortic cannulation by sternotomy conversion is advocated to ensure patient safety in cases of concern or persistent difficulty. Alternative cannulation access, which includes right axillary artery or direct central aortic cannulation, is not advised for the initial PAS learning experience. Peripheral saturation monitoring of the cannulated limb should be mandatory to detect hypoperfusion and the routine use an additional distal perfusion cannula is utilised in some centres.
Atrial exposure and preparation
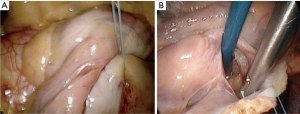
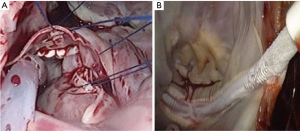
Once safe CPB is ascertained, a longitudinal pericardiotomy is performed above the phrenic nerve, with subsequent exteriorised retraction sutures used to provide unobstructed endoscopic views and working port access of the superior- and inferior vena cava, aorta, atria and interatrial groove (Figure 5A). An additional suspension suture on the intraatrial groove provides additional exposure for an uncomplication left atriotomy. The oblique sinus is exposed and the intraatrial groove developed in preparation for atriotomy (Figure 5B).
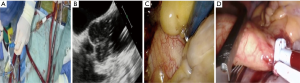
IntraClude™ inflation, antegrade cardioplegia delivery and venting (Figure 6)

Once all team members confirm satisfactory parameters and readiness, the assisting surgeon stabilises the EndoReturn™ cannula with his/her right hand, the IntraClude™ device position is reconfirmed by TEE, partially inflated to approximately 75% of the volume of the ascending aorta and adenosine (0.25 mg/kg) manually syringe-flushed through the device port to achieve rapid diastolic cardiac arrest (Figure 7A). The balloon is then fully inflated under TEE guidance and positioned between the sinotubular ridge and innominate artery (Figure 7B) while antegrade cardioplegia is delivered and monitored by aortic root- and cardioplegic line pressures. The retrograde CPB inflow will push the balloon towards the aortic valve and it is import to pull the device back under TEE guidance to ensure it remains at the sino-tubular junction. It is then locked in position while ensuring satisfactory right arterial line pressures. Endoscopic visualisation and palpation of the aorta with a rigid sucker confirm TEE positioning (Figure 7C). Sudden loss of radial artery trace suggests innominate artery obstruction due to dislodgement and requires rapid repositioning. Even though the safety of current PAS technology compared to direct external aortic clamping strategies utilized in MIAS are well described (42-45), emerging centres should also be familiar with alternative external aortic clamping-, cardioplegia delivery and antegrade venting techniques. Following the placement of a long antegrade venting / cardioplegia needle into the ascending aorta, the transverse sinus is carefully developed with blunt dissection and an external aortic clamp carefully introduced through a separate port to cross-clamp the aorta under endoscopic vision (Figure 7D). The clamp is applied without injuring the pulmonary artery or left atrial appendage. The infrequent application of ventricular fibrillation should also be mentioned. Conversion to sternotomy is strongly advocated if any difficulties are anticipated or occur.
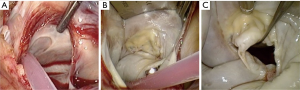
Atriotomy and valve exposure (Figure 8)

Following a generous left atriotomy (Figure 9A), a left atrial retractor with collapsing side arm (USB medical, Hartboro, USA) is inserted into the left atrium, followed by the placement of a cardiotomy vent into the left superior pulmonary vein (Figure 9B). The retractor blade angle can be manually adjusted to ensure unobstructed endoscopic visualization and single shaft instrument access to the mitral valve (MV) and it is recommended to invest adequate time and effort in establishing optimal visualization for procedural ease, especially for suture placement in the anterior MV annulus. Superior- and inferior vena cava occlusion is required for safe right atriotomy and can be achieved with clamps, tape-snares or endoballoon occlusion (Reliant™, Medtronic, Minneapolis, USA) through the internal jugular- and femoral vein respectively. Experienced centres may only use femoral vein cannulation and by retracting the cannula into the inferior vena cava with careful vacuum assisted drainage and flow adjustments, obtain access to the tricuspid valve (TV). It is advocated that emerging centres utilise bicaval venous drainage and safe caval occlusion. Modified TV-retractors or exteriorising sutures can be utilised to obtain easy access and working angles. For intraatrial neoplasm excision, the risk of fragmentation may prohibit the use of an atrial retractor and visualization can subsequently established by traction sutures. In pronounced pectus excavatum deformities, the retractor may be positioned on the left parasternal border. The antero-posterior retraction distance between the right atrium and anterior chest wall may be extremely limited and additional manoeuvers are required to facilitate exposure, which include tilting the patient maximally to the left while applying low positive end-expiratory pressure to the left lung.
Intraatrial and AVV procedures (Figures 10,11)


Large PAS series report the incidence of isolated MV-, isolated TV and combined MV and TV procedures to be 76.0%, 1.9% and 22.1% respectively (40). It is advocated that simple isolated MV or TV procedures are selected during the initial learning experience. The findings of a through intraoperative intraatrial inspection and systematic valve analysis should correlate with the pathology identified by preoperative TEE (Figure 9C). Routine sub-valvular, valvular and annular repair- and replacement procedures can be performed using special long shafted instruments. In cases of MV repair, it is suggested that sub-valvular neochords (Gore-Tex™, Gore & Associates Inc., Arizona, USA) are placed first if required and that annulaplasty suture placement start at P1 progressing up to mid-A2 segment (Figure 12A). Possible IntraClude™ rupture can occur with deep suturing in this zone and requires awareness. Experienced centres advocate the use of additional traction maneuvers, which include a retracting steel wire, to improve MV exposure. Segments P3 to mid-A2 sutures are subsequently placed, followed by the remainder of P1 to P3 (Figure 12B). This sequence prevents distortion of the annulus and allows for perfect exposure. The valve is appropriately sized (Figure 12C) and then parachuted into position. The technique of knot tying deserves special mention (Figure 13A), as long shafted knot-tying devices require continuous suture tension provided by the surgical assistant and good coordination between the surgeon and assistant (Figure 13B). Knotting devices (e.g. Core-knot™, LSI solutions, New York, USA; other options exist) can also be utilised. Experienced centres report a 96.4% MV repair success for primary annular dilatation and degenerative valves, with MV- (Figure 14A) and TV (Figure 14B) repair procedures constituting 82% of experienced PAS program (40). Simple atrial septal defects can be corrected with appropriately sized patch closure (Figure 15A). The base of intraatrial neoplasms is widely excised without manipulation and any defects reconstructed accordingly. Cryoablation for atrial fibrillation (Figure 15B) is performed with an argon-gas surgical ablation system (Medtronic, Minneapolis, USA) prior to annuloplasty suture placement and patent foramen ovale (PFO) routinely closed in 2 layers with a running 3-0 polypropylene suture.

De-airing and procedure conclusion
Once the intended procedure is satisfactory completed, the left superior pulmonary venting catheter is positioned across the MV into the left ventricle and the left atrium closed with two running 3-0 polypropylene sutures from the superior- and inferior incision apex respectively, which is initially snared with the vent across the mitral valve and subsequently tied once the vent is removed. De-airing under TEE guidance is achieved by filling of all cardiac chambers, antegrade aortic root venting through the IntraClude™ device and left ventricular venting through the cardiotomy suction vent in conjunction with continuous CO2 insufflation of the right hemithorax. A temporary epicardial pacing wire is placed on the left ventricular diaphragmatic surface before deflating the IntraClude™ device with the patient in Trendellenburg position. Once general intraoperative parameters are stable and systematic TEE valve- and contractility analysis confirm satisfactory results and are satisfactory, CPB is discontinued and the deflated IntraClude™ device carefully removed. Femoral venous decannulation is performed first, followed by femoral artery decannulation once volume infusions are completed. Post-decannulation femoral artery patency should be confirmed by duplex-Doppler or pulse palpation and any distal perfusion concerns should be immediately addressed by either contrasted angiography and/or reexploration. The cardiotomy suction venting- and atrial retracting incisions are utilised for drainage tubes into the pericardium and hemithorax. The pericardium is subsequently loosely approximated and all wounds sutured in layers to prevent subcutaneous surgical emphysema or lung herniation. Postoperative cardio-respiratory support, sedation, analgesia and other appropriate medication are continued in intensive care, with an individualised in-hospital treatment pathway supervised by a multidisciplinary team.
Overcoming specific learning curves and the potential role of simulation training
Simple atrial septal defect-, intraatrial myxoma- and uncomplicated valve procedures are preferred procedures during the initial learning curve. Hunter (31) identified AVV repair techniques, TEE-guided cannulation, incision placement and setup, transition to single shaft instrument use, AVV visualization and CPB strategies as seven key aspects that contribute to a steep learning curve. The importance of a team consensus on time limit definitions where (49) identified the typical number of operations to overcome the learning curve to range between 75 and 125 procedures and further suggested that more than 1 procedure per week is required to maintain acceptable results. De Praetere (50) reported the learning curve to be 30 procedures, with a significant reduction in aortic cross-clamp time before and after the end of the learning curve. In an era of decreasing surgical volume, simulation team training is strongly advocated (Figure 16).
Future perspectives and conclusion
TC and MIAS technology will continue to evolve and efforts to simplify these platforms will continue as robotic technology-, instrumentation and imaging modalities develop. PAS is proven to be safe and effective for simple and complex AVV surgery and is considered to be a reliable alternative to meet increasing patient demands for less invasive surgical procedures within the context of healthcare trends that aim limits cost-, time- and hospital resources. Experienced centres offer PAS without exclusion criteria, but careful planning and implementation of new programs should focus on risk management and excellent outcomes. Patients are the greatest advocates of a successful PAS program and every effort to reduce adverse outcome risk within a team context should be priority.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Visualized Surgery for the series “Aortic and Mitral Valve Innovative Surgery”. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2019.08.01). The series “Aortic and Mitral Valve Innovative Surgery” was commissioned by the editorial office without any funding or sponsorship. JVDM served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Journal of Visualized Surgery from Feb 2019 to Jan 2021. FC served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Journal of Visualized Surgery from Feb 2019 to Jan 2021. FVP reports personal fees from Edwards, during the conduct of the study; personal fees from Edwards, outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Tully PJ, Baker RA, Turnbull DA, et al. Negative emotions and quality of life six months after cardiac surgery: the dominant role of depression not anxiety symptoms. J Behav Med 2009;32:510-22. [Crossref] [PubMed]
- Schneider EC, Epstein AM. Use of public performance reports: a survey of patients undergoing cardiac surgery. JAMA 1998;279:1638-42. [Crossref] [PubMed]
- Jacobs JP, Certfolio RF, Sade RM. The ethics of transparency: publication of cardiothoracic surgical outcomes in the lay press. Ann Thorac Surg 2009;87:679-86. [Crossref] [PubMed]
- Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: the Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003;24:1231-43. [Crossref] [PubMed]
- Seder CW, Raymond DP, Wright CD, et al. The Society of Thoracic Surgeons General Thoracic Surgery Database 2017 Update on Outcomes and Quality. Ann Thorac Surg 2017;103:1378-83. [Crossref] [PubMed]
- Mack M. Fool me once, shame on you; fool me twice, shame on me! A perspective on the emerging world of percutaneous heart valve therapy. J Thorac Cardiovasc Surg 2008;136:816-9. [Crossref] [PubMed]
- Ferraris VA, Ferraris SP. Assessing the medical literature: let the buyer beware. Ann Thorac Surg 2003;76:4-11. [Crossref] [PubMed]
- Bertrand X. The future of cardiac surgery: find opportunity in change! Eur J Cardiothorac Surg 2013;43:253-4. [Crossref] [PubMed]
- Casselman FP, Van Slycke S, Dom H, et al. Endoscopic mitral valve repair: feasible, reproducible, and durable. J Thorac Cardiovasc Surg 2003;125:273-82. [Crossref] [PubMed]
- Casselman FP, Van Slycke S, Wellens F, et al. Mitral valve surgery can now routinely be performed endoscopically. Circulation 2003;108:II48-54. [Crossref] [PubMed]
- Casselman FP, LaMeir M, Jeanmart H, et al. Endoscopic mitral and tricuspid valve surgery after previous cardiac surgery. Circulation 2007;116:I270-5. [Crossref] [PubMed]
- van der Merwe J, Casselman F, Stockman B, et al. Late redo-port access surgery after port access surgery. Interact Cardiovasc Thorac Surg 2016;22:13-8. [Crossref] [PubMed]
- Pompili MF, Stevens JH, Burdon TA, et al. Port-access mitral valve replacement in dogs. J Thorac Cardiovasc Surg 1996;112:1268-74. [Crossref] [PubMed]
- Pompili MF, Yakub A, Siegel LC, et al. Port-access mitral valve replacement: initial clinical experience (abstr). Circulation 1996;94:I-533.
- Galloway AC, Ribakove GH, Miller JS, et al. Minimally invasive port-access valvular surgery: initial clinical experience (abstr). Circulation 1997;95:I-508.
- Schwartz DS, Ribakove GH, Grossi EA, et al. Minimally invasive cardiopulmonary bypass with cardioplegic arrest: a closed chest technique with equivalent myocardial protection. J Thorac Cardiovasc Surg 1996;111:556-66. [Crossref] [PubMed]
- Schwartz DS, Ribakove GH, Grossi EA, et al. Minimally invasive mitral valve replacement: port-access technique, feasibility, and myocardial functional preservation. J Thorac Cardiovasc Surg 1997;113:1022-30. [Crossref] [PubMed]
- Fann JI, Pompili MF, Burdon TA, et al. Minimally invasive mitral valve surgery. Semin Thorac Cardiovasc Surg 1997;9:320-30. [PubMed]
- Mohr FW, Falk V, Diegeler A, et al. Minimally invasive port-access mitral valve surgery. J Thorac Cardiovasc Surg 1998;115:567-74. [Crossref] [PubMed]
- Galloway AC, Shemin RJ, Glower DD, et al. First report of the Port Access International Registry. Ann Thorac Surg 1999;67:51-6. [Crossref] [PubMed]
- Reichenspurner H. Three-dimensional video and robot-assisted port-access mitral valve operation. Ann Thorac Surg 2000;69:1176-81; discussion 1181-2. [Crossref] [PubMed]
- Falk V. Robot assisted minimally invasive solo mitral valve operation. J Thorac Cardiovasc Surg 1998;115:470-1. [Crossref] [PubMed]
- Grossi EA, Lapietra A, Applebaum RM, et al. Case report of robotic instrument enhanced mitral valve surgery. J Thorac Cardiovasc Surg 2000;120:1169-71. [Crossref] [PubMed]
- Chitwood WR Jr, Nifong LW, Elbeery JE, et al. Robotic mitral valve repair: trapezoidal resection and prosthetic annuloplasty with the da vinci surgical system. J Thorac Cardiovasc Surg 2000;120:1171-2. [Crossref] [PubMed]
- Nifong LW, Chu VF, Bailey BM, et al. Robotic mitral valve repair: experience with the daVinci system. Ann Thorac Surg 2003;75:438-42. [Crossref] [PubMed]
- Atluri P, Stetson RL, Hung G, et al. Minimally invasive mitral valve surgery is associated with equivalent cost and shorter hospital stay when compared with traditional sternotomy. J Thorac Cardiovasc Surg 2016;151:385-8. [Crossref] [PubMed]
- Santana O, Larrauri-Reyes M, Zamora C, et al. Is a minimally invasive approach for mitral valve surgery more cost-effective than median sternotomy? Interact Cardiovasc Thorac Surg 2016;22:97-100. [Crossref] [PubMed]
- Cronin GM, Schroyer M. Financial aspects of building a hybrid operating suite. American Association for Thoracic Surgery, 90th Annual Meeting 2010, 14.06.2011, Available online: http://www.aats.org/2010webcast/sessions/player.html?sid=10050227B.03
- Coddens J, Deloof T, Hendrickx J, et al. Transesophageal echocardiography for port-access surgery. J Cardiothorac Vasc Anesth 1999;13:614-22. [Crossref] [PubMed]
- Gooris T, Van Vaerenbergh G, Coddens J, et al. Perfusion techniques for port-access surgery Perfusion 1998;13:243-7. [Crossref] [PubMed]
- Hunter S. How to start a minimal access mitral valve program. Ann Cardiothorac Surg 2013;2:774-8. [PubMed]
- van der Merwe J, Casselman F, Stockman B, et al. Endoscopic port access surgery for isolated atrioventricular valve endocarditis. Interact Cardiovasc Thorac Surg 2018;27:487-93. [Crossref] [PubMed]
- van der Merwe J, Casselman F, Stockman B, et al. Endoscopic atrioventricular valve surgery in adults with difficult-to-access uncorrected congenital chest wall deformities. Interact Cardiovasc Thorac Surg 2016;23:851-5. [Crossref] [PubMed]
- Van der Merwe J, Casselman F, Stockman B, et al. Endoscopic atrioventricular valve surgery in extreme obesity. Türk Göǧüs Kalp Damar Cerrahisi Dergisi 2017;25:654-8. [Crossref]
- van der Merwe J, Casselman F, Stockman B, et al. Endoscopic Port Access Surgery for Late Orthotopic Cardiac Transplantation Atrioventricular Valve Disease. J Heart Valve Dis 2017;26:124-9. [PubMed]
- van der Merwe J, Casselman F, Van Praet F. Endoscopic Port Access™ left ventricle outflow tract resection and atrioventricular valve surgery. J Vis Surg 2018;4:100. [Crossref] [PubMed]
- Deshpande RP, Casselman F, Bakir I, et al. Endoscopic cardiac tumor resection. Ann Thorac Surg 2007;83:2142-6. [Crossref] [PubMed]
- Van der Merwe J, Van Praet F, Vermeulen Y, et al. Complications and pitfalls in minimally invasive atrioventricular valve surgery utilizing endo-aortic balloon occlusion technology. J Vis Surg 2018;4:248. [Crossref]
- van der Merwe J, Martens S, Beelen R, et al. Total percutaneous cardiopulmonary bypass for robotic- and endoscopic atrioventricular valve surgery. Innovations 2017;12:296-9. [Crossref] [PubMed]
- van der Merwe J, Van Praet F, Stockman B, et al. Reasons for conversion and adverse intraoperative events in Endoscopic Port Access™ atrioventricular valve surgery and minimally invasive aortic valve surgery. Eur J Cardiothorac Surg 2018;54:288-93. [Crossref] [PubMed]
- van der Merwe J, Casselman F, Van Praet F. Endo-aortic balloon inflation under transesophageal echocardiographic guidance. Asvide 2019;6:243. Available online: http://www.asvide.com/watch/32928
- Barbero C, Krakor R, Bentala M, et al. Comparison of Endoaortic and Transthoracic Aortic Clamping in Less-Invasive Mitral Valve Surgery. Ann Thorac Surg 2018;105:794-8. [Crossref] [PubMed]
- Casselman F, Aramendi J, Bentala M, et al. Endoaortic Clamping Does Not Increase the Risk of Stroke in Minimal Access Mitral Valve Surgery: A Multicenter Experience. Ann Thorac Surg 2015;100:1334-9. [Crossref] [PubMed]
- Loforte A, Luzi G, Montalto A, et al. Video-assisted minimally invasive mitral valve surgery: external aortic clamp versus endoclamp techniques. Innovations (Phila) 2010;5:413-8. [Crossref] [PubMed]
- Ius F, Mazzaro E, Tursi V, et al. Clinical results of minimally invasive mitral valve surgery: endoaortic clamp versus external aortic clamp techniques. Innovations (Phila) 2009;4:311-8. [Crossref] [PubMed]
- van der Merwe J, Casselman F, Van Praet F. Restractor placement. Asvide 2019;6:244. Available online: http://www.asvide.com/watch/32929
- van der Merwe J, Casselman F, Van Praet F. Annular sutures. Asvide 2019;6:245. Available online: http://www.asvide.com/watch/32930
- van der Merwe J, Casselman F, Van Praet F. Knotting. Asvide 2019;6:246. Available online: http://www.asvide.com/watch/32931
- Holzhey DM, Seeburger J, Misfeld M, et al. Learning minimally invasive mitral valve surgery: a cumulative sum sequential probability analysis of 3895 operations from a single high-volume center. Circulation 2013;128:483-91. [Crossref] [PubMed]
- De Praetere H, Verbrugghe P, Rega F, et al. Starting minimally invasive valve surgery using endoclamp technology: safety and results of a starting surgeon. Interact Cardiovasc Thorac Surg 2015;20:351-8. [Crossref] [PubMed]
Cite this article as: Van der Merwe J, Casselman F, Van Praet F. The principles of minimally invasive atrioventricular valve repair surgery utilizing endoaortic balloon occlusion technology: how to start and sustain a safe and effective program. J Vis Surg 2019;5:72.