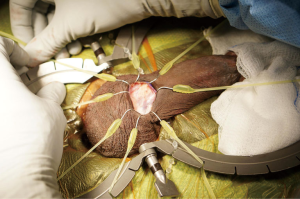
Simultaneous implantation of an inflatable penile prosthesis and an artificial urinary sphincter
Introduction
Erectile dysfunction (ED) and stress urinary incontinence (SUI) are common iatrogenic problems in patients post prostatectomy. According to one study, only 22% of men experience erections firm enough for intercourse at 24 months, and 10% experience frequent urinary leakage or no urinary control, which often deteriorates with time (1).
Additionally, ED and SUI comprise two quality-of-life factors that affect a patient’s decision to delay surgery, as well as patient’s overall long-term satisfaction with the surgery (2,3).
The gold standard for treatment of ED and SUI is the surgical implantation of an inflatable penile prosthesis (IPP) and artificial urinary sphincter (AUS), respectively. Both devices have been shown to dramatically improve patients’ quality of life (4,5). Despite post-prostatectomy patients often presenting for evaluation of both ED and SUI, the simultaneous implantation of both devices remains relatively infrequent and the surgery is primarily limited to a small number of high-volume surgeons.
Previous literature suggested that dual implantation led to an increased rate of infection and mechanical failure; however, more up-to-date data demonstrate that, with the new and advanced antibiotic-coated devices, infection rates are the same as documented for single prosthetic implantation (6). Given the significant savings in time, cost, and convenience to the patient, synchronous dual-implantation can be considered for patients who would benefit from both IPP and AUS placement.
In this article, we offer our technique for simultaneous placement of an AUS and an IPP, with special attention given to the management of potential pitfalls. In trained hands, we believe this to be a safe and efficacious way to manage concomitant ED and SUI in appropriately selected patients, while limiting anesthetic exposure.
Surgical technique (Figure 1)

When considering simultaneous placement of an IPP and AUS, patient selection is of utmost importance. At our institution, we follow the same strict criteria for dual implantation that is followed for either AUS or IPP placement alone. Diabetic patients are delayed until there is a decrease in their hemoglobin A1c to a level of 7 or lower. A negative preoperative urine culture is mandatory.
It is critical that the physical exam is done in the supine position to determine the approach. If the same location on the bulbar urethra is palpable from a penoscrotal approach and a perineal approach, the patient may be a good candidate for a single incision dual implant. This examination should be repeated at the time of surgery to verify this approach. Performing a perineal AUS followed by a penoscrotal or infrapubic IPP is a reasonable alternative to a single incision dual implantation, as it uses a counter incision to place the pressure-regulating balloon (PRB).
The patient is then prepped and draped in the usual sterile fashion for prosthetics cases. We administer perioperative antibiotics in accordance with the AUA Best Practices Statement (8). A 12-French Foley catheter is placed to identify the urethra without affecting the circumferential measurement later in the case. A transverse incision is made just below the penoscrotal junction, and a Scott retractor is positioned to optimize exposure for the remainder of the procedure (see Figure 2). The urethra is then dissected out using a combination of sharp and blunt dissection, with the surgeon targeting the most proximal aspect of the urethra he or she can access. If dissection proves to be difficult (secondary to a large amount of tissue between the skin and the urethra), a Babcock clamp can be placed around the urethra, using the Foley catheter as guidance, to aid in the urethral dissection. A peanut can be used to atraumatically dissect the posterior aspect of the urethra. If there is any concern for an injury to a posterior urethra, a retrograde urethrogram or a cystoscopy is performed. In the event that a urethral injury is identified, the prosthetic case is aborted.
Once a right-angle clamp can safely pass around the posterior aspect of the urethra, the measuring tape is used to measure the circumference of the urethra. In the interest of efficiency, the PRB can be placed while the cuff is being prepared.
After confirmatory emptying of the bladder, the surgeon tracks up the inguinal canal and then pierces through the medial floor of the canal just superior to the pubic bone. For the PRB, this is conventionally done on the patient’s right side, with the opening made only large enough to fit the deflated balloon. If the defect is too large, there is a chance of PRB herniation. If this is a concern, a counter incision can be utilized with securing sutures to minimize this risk. Once the PRB is positioned, it is filled with 23–26 cc of fluid and clamped with a shod. Be aware that backpressure is expected when using a PRB.
The cuff is then positioned by placing a right-angle clamp behind the urethra. The clamp should enter the same side as the PRB tubing. The cuff, not the tubing, should then be passed into the clamp and positioned around the urethra. This orientation prevents the tubing from crossing over the urethra.
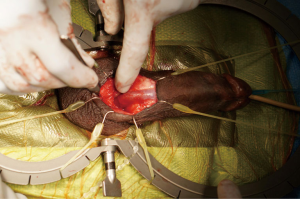
Attention is then turned to the scrotal pump, which is first secured to the PRB and the cuff using the quick connect system provided by the manufacturer. A sub-dartos scrotal pouch is then created digitally in a superficial and dependent location. The pump is navigated into the space and secured by placing a Babcock clamp on the tubing. The pump is then further secured with interrupted absorbable purse-string suture. The incision is then closed in layers to cover all components of the AUS (Figure 3).
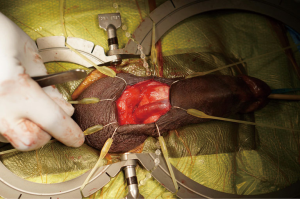
Without closing the skin, the Scott retractor is positioned towards the penis for placement of the penile prosthesis (Figure 4). Once the ventral corpora are dissected bilaterally, two sets of 2-0 absorbable sutures are placed medially and laterally on each corpus. The second set of stay sutures is placed proximal to the first (total of eight stay sutures placed).
The corporotomy incisions are then made between the stay sutures, and the intracorporal space is dilated sequentially from 10 to 14 mm. Each corpus is individually measured proximally and distally in order to size the device properly. Once the cylinder optimal length is determined, attention is turned to the reservoir. If there is a significant discrepancy (>1 cm) between the left and right side, crossover or perforation should be suspected and assessed by performing a “field goal post” test proximally and distally, with a dilator in each corpus. The dilator should be oriented parallel to one another and be of similar length.
The reservoir is ideally placed on the side opposite (left) the PRB. A similar approach is taken for the prosthesis reservoir. Again, a puncture is made in the transversalis fascia (usually with Mayo scissors), and the reservoir is navigated into that space digitally. The reservoir is then filled with the appropriate amount of injectable saline, which is determined by the size and model of the penile prosthesis. In the case of the prosthetic reservoir, backpressure is not expected when it is positioned correctly.
The cylinders are then placed with the aid of a Furlow inserter. The Furlow inserter allows for distal positioning in a dorsal and lateral position on the glans. The proximal aspect of each cylinder is placed, followed by the distal part of each cylinder.
A surrogate reservoir (60 cc syringe) is then used to fill the cylinders with saline to assess the device for cosmetic result, glanular support, symmetry, and curvature. If undiagnosed Peyronie’s disease is identified, straightening maneuvers can be performed; however, this is outside of the scope of this review. The cylinders are then deflated, and once the positioning is deemed adequate, the corporotomies are closed by tying the previously placed stay sutures over the incision in an interrupted fashion from the most distal to the most proximal.
The reservoir is then connected to the cylinders and pump using the connection system provided by the manufacturer. A space is then made digitally in a posterior and dependent location. The scrotum is everted, and the pump navigated into the newly created pouch. Again, the pump is secured by placing a single absorbable suture (Figure 5). The incision is then closed in layers and the skin covered with skin glue. Xeroform™ gauze (Cardinal Health, Dublin, OH, USA) is placed over the incision, and the shaft of the penis is wrapped in a compressive dressing. A Kerlix™ (Cardinal Health, Dublin, OH, USA) dressing is then placed around the scrotum and penis.
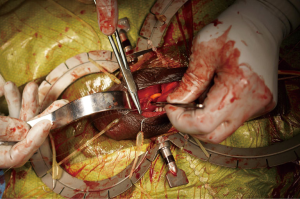
Patients are sent home or kept in the hospital overnight as outpatients for intravenous (IV) antibiotics. The Foley catheter is removed on the following morning, and the devices are activated in a staged fashion starting 5 to 6 weeks after surgery.
Discussion
ED and urinary incontinence often occur in tandem. In recent years, there have been multiple studies of dual implants in a single operative setting. There has been some concern that dual implantation may increase the rate of infection; however, no increased rates of adverse events, erosion, malfunction or infection in a single operation dual implant has been documented (9). One series on dual synchronous implantation using two incisions demonstrated a higher rate of reoperation (0.98 per patient) with 11% of patients suffering erosion or infection, using older prosthesis models (10) Kendirci et al. demonstrated a revision rate of only 14% with two patients of 17 suffering from cuff erosion (11). Rolle et al. performed the synchronous procedures through a single scrotal incision and had only one infection; this was after an endoscopic procedure on a patient who had undergone simultaneous dual implant (12). One of the most extensive retrospective studies comparing single implant of AUS or IPP compared to synchronous dual implant showed no increased peri-operative infection rates or decreased device survival (13). Single setting for dual implantation has also been shown to reduce overall operative time and cost (14). The outcomes concerning patient satisfaction rival that of each procedure individually, with most patients preferring a single operation (11,15).
Limitations
Although the technique of dual implants has been previously reported for some years, most series have all been in the hands of high-volume prosthetic surgeons. Further, the data is almost exclusively retrospective reviews. Due to the nature of these studies (also done almost exclusively by high-volume providers), their reproducibility has not been performed.
Conclusions
Simultaneous implantation of an IPP and an AUS is a safe and effective management strategy for men with concomitant ED and SUI. While this procedure is more complex than a staged approach, our experience and a review of the literature has not revealed the complication rate to be any higher. Thus, in experienced hands, dual implantation is a safe approach with the added benefit of limiting anesthetic exposure.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Martin Gross, Jay Simhan and Faysal A. Yafi) for the series “Penile Prosthesis Surgery” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2019.07.08). The series “Penile Prosthesis Surgery” was commissioned by the editorial office without any funding or sponsorship. WJGH reports personal fees from Coloplast (consultant/advisor), personal fees from Boston Scientific (consultant/advisor), outside the submitted work. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. At the time the surgical consent was obtained, the patient also gave consent for photography and videography for educational purpose.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Penson DF, McLerran D, Feng Z, et al. 5-year urinary and sexual outcomes after radical prostatectomy: results from the prostate cancer outcomes study. J Urol 2005;173:1701-5. [Crossref] [PubMed]
- Prabhu V, Lee T, McClintock TR, et al. Short-, Intermediate-, and Long-term Quality of Life Outcomes Following Radical Prostatectomy for Clinically Localized Prostate Cancer. Rev Urol 2013;15:161-77. [PubMed]
- Abraham NE, Makarov DV, Laze J, et al. Patient centered outcomes in prostate cancer treatment: predictors of satisfaction up to 2 years after open radical retropubic prostatectomy. J Urol 2010;184:1977-81. [Crossref] [PubMed]
- Kahlon B, Baverstock RJ, Carlson KV. Quality of life and patient satisfaction after artificial urinary sphincter. Can Urol Assoc J 2011;5:268-72. [Crossref] [PubMed]
- Bettocchi C, Palumbo F, Spilotros M, et al. Patient and partner satisfaction after AMS inflatable penile prosthesis implant. J Sex Med 2010;7:304-9. [Crossref] [PubMed]
- Christine B, Knoll LD. Simultaneous advance male sling and an inflatable penile prosthesis: concurrent placement does not increase potential for implant infection. J Urol 2011;185:e726 [Crossref]
- Tsambarlis PN, Koller C, Leinwand G, et al. Dual implantation of an artificial urinary sphincter and inflatable penile prosthesis. Asvide 2019;6:225. Available online: http://www.asvide.com/watch/32910
- Wolf JS Jr, Bennett CJ, Dmochowski RR, et al. Best practice policy statement on urologic surgery antimicrobial prophylaxis. J Urol 2008;179:1379-90. [Crossref] [PubMed]
- Segal RL, Cabrini MR, Harris ED, et al. Combined inflatable penile prosthesis-artificial urinary sphincter implantation: no increased risk of adverse events compared to single or staged device implantation. J Urol 2013;190:2183-8. [Crossref] [PubMed]
- Parulkar BG, Barrett DM. Combined implantation of artificial sphincter and penile prosthesis. J Urol 1989;142:732-5. [Crossref] [PubMed]
- Kendirci M, Gupta S, Shaw K, et al. Synchronous prosthetic implantation through a transscrotal incision: an outcome analysis. J Urol 2006;175:2218-22. [Crossref] [PubMed]
- Rolle L, Ceruti C, Sedigh O, et al. Surgical implantation of artificial urinary device and penile prosthesis through trans-scrotal incision for postprostatectomy urinary incontinence and erectile dysfunction: synchronous or delayed procedure? Urology 2012;80:1046-50. [Crossref] [PubMed]
- Boysen WR, Cohen AJ, Kuchta K, Park S, Milose J. Combined Placement of Artificial Urinary Sphincter and Inflatable Penile Prosthesis Does Not Increase Risk of Perioperative Complications or Impact Long-term Device Survival. Urology 2019;124:264-70. [Crossref] [PubMed]
- Sellers CL, Morey AF, Jones LA. Cost and time benefits of dual implantation of inflatable penile and artificial urinary sphincter prosthetics by single incision. Urology 2005;65:852-3. [Crossref] [PubMed]
- Mancini JG, Kizer WS, Jones LA, et al. Patient satisfaction after dual implantation of inflatable penile and artificial urinary sphincter prostheses. Urology 2008;71:893-6. [Crossref] [PubMed]
Cite this article as: Tsambarlis PN, Koller C, Leinwand G, Hellstrom WJG. Simultaneous implantation of an inflatable penile prosthesis and an artificial urinary sphincter. J Vis Surg 2019;5:69.