Minimally invasive cardiac surgery: state-of-the-art right heart surgical technique for tricuspid valve, atrial septal defect and myxoma
Introduction
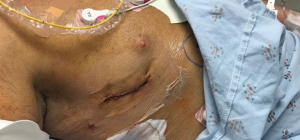
The most common adult right heart surgery are tricuspid repair and replacement, atrial septal defect (ASD) closure and myxoma resection. All of the above procedures are done mostly via the right atrium. Minimally invasive heart surgery has become the standard open-heart surgery at many main academic centers. Various reports have been published and have shown the benefits of minimally invasive heart surgery (1-7). The benefits include shorter ICU and hospital stay, faster recovery, earlier patient ambulation, lower blood transfusion requirement, reduced post-op atrial fibrillation rate, decreased wound infection rate and less hospitalization cost (1-7). In minimally invasive heart surgery, the surgeon performs heart surgery through a small incision (Figures 1,2), without sternotomy, under direct vision utilizing specialized instrumentation. Right atrium can easily be assessed via a right lateral mini-thoracotomy through a 4–5 cm incision within the 4th–5th intercostal space. Minimally invasive heart surgery can have a hilly learning curve and surgeons should pay special attention at each step of the procedure. In this report, we attempted to provide some tips and techniques for minimally invasive right heart surgery.
Patient selection
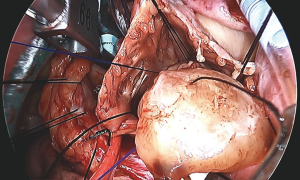
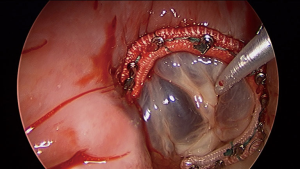
In general, minimally invasive heart surgery can be performed on most patients including patients with tricuspid valve disease (Figure 3), ASD (Figure 4) and myxoma (Figure 5). Double or triple valves surgery can also be performed minimally invasively (4), for example patients with both mitral and tricuspid valve disease which could be a common scenario. All patients with primary or secondary tricuspid regurgitation are candidates for minimally invasive heart surgery and both tricuspid repair and replacement can be done effectively. Minimally invasive heart surgery is applicable to all types of ASD, including the most common secundum ASD and sinus venosus ASD. Both left atrial myxoma and right atrial myxoma can be done minimally invasively. For patients with prior heart surgery, minimally invasive heart surgery using a right mini-thoracotomy to avoid a redo-sternotomy has made open heart surgery less invasive and less traumatic for patients. Peripheral cannulation for patients with severe peripheral vascular disease may be prohibitive, but axillary cannulation can be performed as an alternative. From our experiences, minimally invasive heart surgery is also beneficial for patients with morbid obesity and has allowed a faster recovery, earlier ambulation, shorter ICU and hospital stay and no sternal complication. For tricuspid surgery, minimally invasive heart surgery can be performed on a beating heart. The usual cardiac surgery preoperative screening is obtained prior to surgery. CT scan is not necessary for all patients, unless patient has a risk of or have peripheral vascular disease.
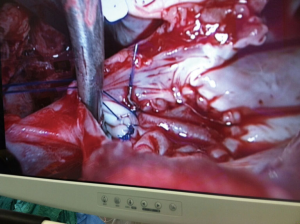
Surgical technique
General anesthesia is used in minimally invasive heart surgery. A radial arterial line is placed in the standard fashion. Swan-Ganz catheter can be placed if needed. Defibrillation pads should be placed on the patient’s left chest and right shoulder. A single-lumen endotracheal tube is preferred, and ventilation can be paused after the initiation of cardiopulmonary bypass. Single-lung ventilation should be avoided to reduce the risk of unilateral pulmonary edema post-op. An intraoperative transesophageal echocardiogram is utilized to assess tricuspid valve pathology and anatomy, ASD location and size or myxoma location and size. A sheet roll is placed under the patient right chest for chest elevation, and the right arm is positioned at a 45 degree angle off to the side of the bed. The patient’s chest, abdomen and pelvis are prepped and draped in the standard surgical fashion. The surgical technique for minimally invasive right heart surgery is similar to the technique we published previously for minimally invasive mitral surgery with some modifications (5). Femoral artery and vein are generally used for cannulation. For patients with iliac disease or small and calcified femoral arteries, axillary artery may be used for cannulation. Seldinger technique with transesophageal echocardiogram guidance is used for femoral artery and vein cannulation. It is important to confirm the wire location within the superior vena cava (SVC) prior to inserting the venous cannula toward the right atrium via the femoral vein. We generally perform limited dissection and expose only the anterior aspect of the femoral vessels before cannulation in order to avoid lymphatics injury and lymphocele formation postoperatively. For right heart surgery, bicaval cannulation is needed. A multi-staged femoral venous cannula size 21–25 F should be used with the tip of the cannula advanced to the right atrium-inferior vena cava (IVC) junction. Percutaneous right internal jugular venous cannulation (15 F) will also be needed for bicaval cannulation with the cannula tip placed in the SVC with transesophageal echocardiogram guidance. Direct SVC cannulation can also be performed without difficulty via the right mini-thoracotomy depending on the surgeon preference. A single femoral bicaval cannula can be used with vacuum-assist and is available in the market. But visualization could be difficult during ASD closure or myxoma resection.
A 4 cm incision at the 4th or 5th intercostal space, at or lateral to the anterior axillary line, is made, depending on whether mitral valve surgery is also needed. For female patient, the incision is made laterally at the mammary crease and dissected up toward the 4th or 5th intercostal space. Ioban strips is used to retract the breast medially and superiorly if needed. A rib retractor and soft tissue retractor are utilized for exposure. Extensive rib spreading should be avoided and is not needed, in order to minimize rib fractures and postoperative pain. Cardiopulmonary bypass is initiated, and mechanical ventilation is withheld. The pericardium is opened longitudinally about 3 cm above the phrenic nerve. Pericardial retraction sutures are placed and exited through the skin in order to improved exposure. Both SVC and IVC are dissected and separated from surrounding structures to allow occlusion before right atrium is open. It should be noted that dissection and occlusion of the SVC and IVC is not always needed, if vacuum assist and experienced perfusionist are available. If aortic cross-clamping is planned, a purse-string suture is placed in the mid-ascending aorta for cardioplegia/root vent cannula insertion. Ventricular fibrillation arrest can be used in the standard fashion for ASD closure and myxoma resection, in patients with calcified ascending aorta or in redo heart surgery, where aortic cross-clamp are not permitted. For isolated tricuspid valve surgery, beating heart surgery without cardiac arrest can easily be performed minimally invasively. If there is significant aortic regurgitation in cardiac arrest cases, a retrograde cardioplegia catheter can be placed into the coronary sinus ostium after the right atrium is open. For the cardiac arrest case, flexible aortic cross clamp is used. Antegrade cardioplegia is administrated. A vertical or horizontal right atriotomy incision can be made depending on surgeon preference. For tricuspid surgery, we generally use a horizontal incision and for ASD or myxoma we use a vertical incision. If mitral valve surgery is needed concomitantly, mitral valve surgery should be performed first via the AV groove before right heart surgery. An atrial lift retractor blade is inserted into the right atrium. With proper placement of the atrial retractor, great and direct visualization of the tricuspid valve apparatus and atrial septum can be accomplished (Figures 3-5). Upon completion of the right heart surgery, the atriotomy is closed with 4-0 prolene suture in a running two-layer fashion. A right ventricular pacing wire is placed at this time. Cardiopulmonary bypass is weaned. Protamine is administrated and decannulation is performed in the usual fashion. Chest tubes are placed within the pericardial space and in the right pleural space. The chest and groin are closed in the usual fashion.
Tricuspid valve surgery
Both tricuspid valve repair (Figure 3) and replacement can be performed minimally invasively depending on the tricuspid pathology, e.g., endocarditis, rheumatic disease or secondary tricuspid regurgitation. Standard tricuspid repair and replacement technique should be applied with the avoidance of the conduction system within the triangle of Koch. For repair, we generally place sutures first around the annulus and avoiding part of the septal annulus to further improve visualization. Leaflet resections, chord placement or translocation and annuloplasty can be easily performed under direct vision. Previous mitral valve surgery or coronary artery grafting are not a contraindication for minimally invasive tricuspid valve surgery. From our experiences, minimally invasive right heart surgery is less traumatic to the patient than a redo-sternotomy which will allow faster recovery for the patient and less blood transfusion requirement. Tricuspid regurgitation, despite national guideline (8,9), is commonly under treated with delayed intervention until patients have liver cirrhosis and right heart failure. Tricuspid valve regurgitation, both primary and secondary regurgitation, should be intervened early to avoid irreversible liver failure, renal failure, right heart dilation and failure. Patient with symptoms such as ascites, lower extremities edema, generalized fatigue, pulmonary hypertension should be intervened early to improve quality of life and to avoid irreversible organs failure. Besides symptoms, it has been recommended that a tricuspid annulus diameter greater than 40 mm, atrial fibrillation and signs of right heart failure, tricuspid repair should be considered. Minimally invasive double valve surgery, e.g., mitral valve and tricuspid repair can be performed. For patient undergoing mitral surgery, tricuspid repair or replacement is universally suggested in the presence of severe tricuspid regurgitation or tricuspid annular dilation because tricuspid regurgitation does not resolve spontaneously after mitral valve surgery as some believed.
ASD closure
All types of ASD can be repaired and closed with minimally invasive approach including the most common sinus venous ASD (Figure 4) and secundum ASD. For sinus venosus ASD, additional dissection of the distal SVC may be needed to allow enough room for the sinus venosus ASD to be repaired and the occlusion of the distal SVC. Right internal jugular vein percutaneous venous cannulation is recommended in addition to the femoral vein cannulation. Warden procedure can also be performed minimally invasive if needed. Examination of the right pulmonary veins is needed to avoid obstruction. And SVC syndrome should be avoided either with a patch repair technique or Warden procedure if needed. Both can be done minimally invasively.
Myxoma resection
Left atrial myxoma resection (Figure 5) can be performed in the standard fashion via the transseptal approach or through the left atrium. Complete resection of the tumor is important to avoid recurrence. A thoracoscope is used to obtain a closer image to assure complete resection. A patch repair of the ASD is often needed after myxoma resection and can be done minimally invasively as described above. For large myxoma, an endoscopic specimen bag can be used to retrieve the mass out of the chest and via the small mini-thoracotomy incision to avoid lose pieces and contamination.
Conclusions
Minimally invasive right heart surgery is feasible and can be performed through a small incision (4–5 cm) without sternotomy or rib cutting. Multiple reports have been published and proven its benefits. Most cardiac surgeries can be performed minimally invasive including right heart surgery. The most common adult right heart surgeries are tricuspid surgery, ASD closure and myxoma resection. Minimally invasive heart surgery can be surgically demanding, cautions and attention to details are needed to perform the procedure safely and effectively. Patients will benefit from the success of the operation performed by a surgeon with a good foundation and knowledge base of minimally invasive heart surgery.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Journal of Visualized Surgery for the series “Minimally Invasive Cardiac Surgery”. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2019.07.01). The series “Minimally Invasive Cardiac Surgery” was commissioned by the editorial office without any funding or sponsorship. AC served as the unpaid Guest Editor of the series and serves as an unpaid editorial board member of Journal of Visualized Surgery from Jun 2015 to May 2021. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ricci D, Boffini M, Barbero C, et al. Minimally invasive tricuspid valve surgery in patients at high risk. J Thorac Cardiovasc Surg 2014;147:996-1001. [Crossref] [PubMed]
- Ellouze M, Pellerin M, Jeanmart H, et al. Mini Right Anterior Thoracotomy Approach Versus Sternotomy for Resection of Intracardiac Myxoma. Innovations (Phila) 2018;13:292-5. [Crossref] [PubMed]
- Galloway AC, Schwartz CF, Ribakove GH, et al. A decade of minimally invasive mitral repair: long-term outcomes. Ann Thorac Surg 2009;88:1180-4. [Crossref] [PubMed]
- Santana O, Xydas S, Williams RF, et al. Outcomes of minimally invasive double valve surgery. J Thorac Dis 2017;9:S602-6. [Crossref] [PubMed]
- Cheng A, Ramsey AM. Minimally invasive mitral valve surgery: tips, tricks and technique. J Vis Surg 2018;4:182. [Crossref]
- Iribarne A, Easterwood R, Russo MJ, et al. Comparative effectiveness of minimally invasive versus traditional sternotomy mitral valve surgery in elderly patients. J Thorac Cardiovasc Surg 2012;143:S86-90. [Crossref] [PubMed]
- Moscarelli M, Fattouch K, Casula R, et al. What Is the Role of Minimally Invasive Mitral Valve Surgery in High-Risk Patients? A Meta-Analysis of Observational Studies. Ann Thorac Surg 2016;101:981-9. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Thorac Cardiovasc Surg 2014;148:e1-132. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017;135:e1159-95. [Crossref] [PubMed]
Cite this article as: Cheng A, Ramsey AM. Minimally invasive cardiac surgery: state-of-the-art right heart surgical technique for tricuspid valve, atrial septal defect and myxoma. J Vis Surg 2019;5:63.