Minimally invasive mitral valve repair for Barlow’s syndrome with functional prolapse using artificial chords
Mitral valve (MV) disease is one of the most common reasons for valve related cardiac surgery, MV prolapse affects 3% of the population (1,2). Various advances in understanding the MV over the past decades have led to promotion of early intervention, which is recommended in the 2017 European Society of Cardiology/European Association for Cardio-Thoracic Surgery guidelines for the management of valvular heart disease (3). The 2017 AHA/ACC guidelines advocate an early treatment of MV disease even more pronounced (4). Three basic principles are described by Alain Carpentier and colleagues: restoring or preserving the mobility of both leaflets, the importance of a large surface of coaptation, and remodeling the diseased MV annulus (5). Today, MV repair is the gold standard for the treatment of significant MV regurgitation (MVR) (6).
Surgical approach
Besides standard of care full sternotomy different minimally invasive techniques are established, to decrease surgical trauma and accelerate postoperative recovery, thus increasing acceptance of MV surgery.
The mini-sternotomy approach is mainly used for aortic valve and MV surgery (7,8). We use this technique especially in patients with severely calcified MV annulus to facilitate excellent exposure and surgical handling still offering less invasive treatment (9,10).
The right mini-thoracotomy approach is successfully used by many groups, already in 2003 Casselman and colleagues demonstrated feasibility and durability of so called endoscopic MV repair (11). Many surgeons use this technique under direct vision. With the development of 3D endoscopes totally endoscopic MV surgery with even smaller incisions is feasible representing the least invasive surgical approach today (12,13).
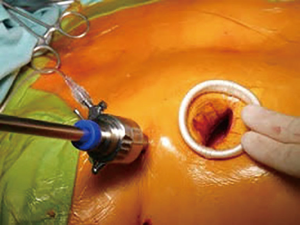
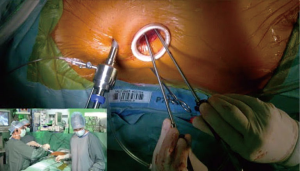
At our center, since the introduction of mini-thoracotomy approach for MV surgery in 2001 exactly 600 patients were operated under direct vision and since the implementation of a 3D endoscope in 2015 another 295 patients underwent fully endoscopic MV surgery. The typical setup at our center consists of a peri-areolar incision in male and a sub-mammary incision in female patients, in all cases entering the fourth intercostal space. All other instruments (transthoracic aortic clamp, left heart vent, cardioplegia line and left atrial retractor) are introduced via the third intercostal space. Extracorporeal circulation is predominantly established via cannulation of the groin vessels, in rare cases of heavily calcified abdominal aorta the axillary or carotid artery is cannulated (Figures 1-6).
Barlow’s syndrome
The most prevalent cause of MVR is degenerative MV disease. In the variety of degenerative MV disease Barlow’s syndrome is a spectrum of phenotypes with its extreme form manifesting typically in young patients (14). Barlow’s syndrome is a clinical syndrome, Barlow and colleagues primarily described auscultatory-electrocardiographic findings (15). Subsequently, four patients were described presenting with the typical auscultatory murmurs in whom the posterior leaflet underwent massive protrusion into the left atrium during systole (16). Histological examination of valve tissue resected during MV replacement in similar patients showed severe myxomatous degeneration of leaflets and chordae. Also, the term “billowing” was used to morphologically describe the valve leaflet motion (17). Further on, the valve pathology was described as “floppy valve” or “myxomatous valve”. Carpentier defined the syndrome as a bi-leaflet prolapse, a billowing valve with excessively thickened leaflets and severe annular dilatation. Additionally, calcification of the leaflets or the annulus is common in late disease (18). Due to the complex, multi-segmental changes of the valve and the lack of normal tissue to serve as a reference point MV repair was considered as difficult and frequently ended up in MV replacement (19,20). In addition to excessive tissue as a challenge for repair per se the size of the leaflets predisposes the patients for a higher risk of a systolic anterior motion (SAM) phenomenon (21).
Reconstructive concepts in Barlow’s syndrome
Nevertheless, various reconstructive approaches are described and successfully used in this pathology.
After exposure and valve analysis one common issue is to address mitral annular calcification (MAC) which is present in around 20% of patients. A calcium bar usually encased in a fibrous capsule can often be resected in toto. Subsequently, valve repair is carried out (22).
Jouan and colleagues from Carpentier’s working group applied a quadrangular resection of the posterior leaflet in most patients presenting with Barlow’s syndrome. It was performed without a posterior sliding leaflet plasty in 29.7%, and with a sliding leaflet plasty in 69.8%. By this procedure the height of the posterior leaflet was reduced effectively thus avoiding the risk for SAM, which occurred in very few patients in this cohort. Commissural prolapse was addressed by commissuroplasty (localized edge-to-edge technique). According to the principles of MV repair an annuloplasty ring was implanted in all patients, the predominant sizes of the ring were 36 mm or bigger. Results were promising with long-lasting repair rates (23). The presence of anterior leaflet billowing or prolapse was treated with papillary muscle plasty or an Edge-to-edge repair (23,24).
In contrast, Miura et al. describe a triangular resection and chordal replacement as “restoration technique”. In a small number of patients, the triangular resection was successfully applied to correct the posterior as well as the anterior leaflet. This concept addresses not only the excessive height of leaflets but also the extended width, which occurs as a consequence of degeneration (25). The reduction of the anterior leaflet size by resection contributed to the 100% successful prevention of SAM in this series.
A similar technique was reported by Fasol at al. In addition to a quadrangular resection of the P2 segment with sliding leaflet plasty of the remaining P1 and P3 segments together with plication of the posterior annulus, a triangular resection of the anterior leaflet in the A2 region was performed. An annuloplasty ring was implanted to restore annular geometry. The results were promising with no recurrent MVR during long-term follow-up (26).
An alternative to resection of valve tissue is to address the subvalvular apparatus in order to restore leaflet coaptation (27). Several techniques on the level of native chordae (chordae shortening or chordae transfer) or papillary muscles (papillary muscle plasty or relocation) are described and used successfully in MV repair, even in the field of Barlow’s syndrome (5,22). During the last decade artificial chordae (either single sutures or pre-measured loops) consisting of expanded polytetrafluorethylene (ePTFE) are liberally used, not only in the setting of isolated P2 prolapse but also in Barlow’s syndrome. In many centers, implantation of ePTFE chordae replaced sophisticated surgical techniques on the level of the papillary muscles or native chordae (13,19,28-30). Artificial chordae can be implanted rather easily, also in the setting of minimally invasive MV surgery (10,13). The appropriate length of chordae treats the leaflet prolapse successfully and minimizes the risk for SAM.
Instead of dealing with the size of leaflets or the appropriate length of chordae the so called “Alfieri-Technique” (edge-to-edge repair) also was analyzed by the Leipzig group in the context of minimally invasive repair. Either implantation of neochordae or the edge-to-edge technique were applied to Barlow’s disease patients in addition to annuloplasty. The Alfieri-technique was feasible and allowed for shorter operative times but resulted in mildly increased transvalvular gradients, however still in a non-stenotic range (19). Although the edge-to-edge stich appears to be easily performed an exact placement at the proper site of leaflets is mandatory to achieve good results (19,24).
A common complication during valve repair in Barlow’s syndrome is the development of a SAM phenomenon (29). Reducing the height of the posterior leaflet either by tissue resection or by implantation of appropriately short neochordae prevents the anterior leaflet from being displaced towards the left ventricular outflow tract during systole (5,21). Another mechanism for development of SAM is an inappropriately small annuloplasty ring forcing excessive tissue into the left ventricular outflow tract. The latter can be overcome by using large, at least true-sized if not over-sized annuloplasty rings. Adams and colleagues report on a series of Barlow’s disease patients in whom annuloplasty rings no smaller than 36 mm were used resulting in 100% absence of SAM (21).
All studies report good results and at least acceptable long-term durability of the used repair technique. Nevertheless, an “optimal” strategy for Barlow disease patients is not defined. Castillo and colleagues report on the use of different techniques, such as chordal transfer or transposition, implantation of neochordae, commissural sutures or triangular resection. This “blend of approaches” results in 90% freedom from moderate MVR at 7 years (20). Most probably the use of a widespread armamentarium reflects the best practice to deal with the complex pathology in Barlow’s syndrome and a distinct “best method” cannot be applied successfully to each patient. At our center we also use various techniques for the repair of bileaflet prolapse including the mentioned measures. We implant an annuloplasty ring in all patients, according to Adams’ findings we always use large annuloplasty rings with a tendency to oversize the prosthetic ring.
In numerous centers the surgical approach for Barlow’s syndrome patients is the median sternotomy to address the complex pathology and allow for sophisticated repair. With the previously described advances of minimally invasive MV surgery even bileaflet prolapse valves can be successfully repaired with either partial sternotomy or right mini-thoracotomy (5,10). In a randomized trial, Speziale and colleagues demonstrated reproducible repair results in the minimally invasive setting under direct vision. They advocate for minimally invasive approach even in complex settings such as Barlow’s syndrome (31). In our own center we perform a routine CT scan in each patient referred for MV surgery. In case of MAC the patient is scheduled for partial sternotomy. This allows for complete resection of MAC and annular repair by atrial sliding plasty if required and subsequent reconstruction of the MV. All other patients, including Barlow disease patients, meanwhile undergo a fully endoscopic MV repair using a 3D video endoscope.
Subgroup of patients—functional prolapse
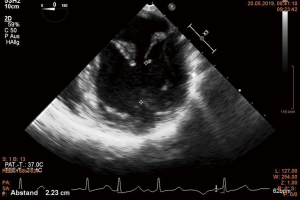
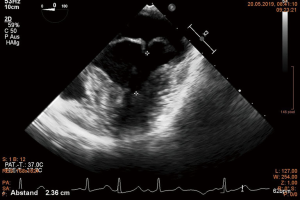
Several working groups report on a subgroup of Barlow’s syndrome patients in whom only a ring annuloplasty was successfully performed (14,32-36). The term “functional prolapse” was introduced. Although Barlow disease involves the whole MV apparatus, the mitral annulus plays a significant role in the development of MVR (36). The area encircled by the annulus is significantly enlarged in comparison to normal subjects and the physiologic annular motion is impaired (32). Normally, in early systole antero-posterior contraction at the level of the MV annulus accentuate the saddle shape of the annulus (37). This movement brings the two leaflets together to avoid early systolic MVR. The locking together of the leaflets allows the tips of the papillary muscles to move towards the apex pulling the free edge of the leaflets into the left ventricular cavity further enhancing coaptation and reduction of chordal tension during systole (38,39). The loss of these features promotes leaflet separation. Moreover, the prolapsing leaflets exert traction on papillary muscles, which now move paradoxically towards the left atrium rather than towards the left ventricular apex (38). This leads to a reduced distance between the papillary muscle tip and the annular plane during systole (32). In addition, a left ventricular wall motion abnormality was described already by Ehlers and associates. A “systolic contraction ring” at the level of the bases of the papillary muscles was observed. It was hypothesized that this could lift the papillary muscles toward the MV annular plane during systole causing the chordae to slacken (40). These findings were confirmed by others and summarized by Lawrie (34). This movement can be visualized in echo, especially the abnormal wall motion at the basis of the papillary muscles can be observed. The paradox movement of the papillary muscles is demonstrated by almost equal distance between the muscle tip and the free edge of the prolapsing leaflet during systole and diastole (Figures 7,8). During surgery, a water test performed on the cardioplegic heart before reconstruction showing no significant regurgitation in the cardioplegic heart finally proves the diagnosis of functional prolapse (35,36). In contrast, a prolapse on the basis of ruptured or severely elongated or ruptured chordae would be apparent during water testing. It has to be emphasized that any additional “true” i.e., anatomical prolapse has to be corrected in routine fashion in addition to annuloplasty (34).
Recently, an additional pathologic pattern of the MV annulus was focused: mitral annular disjunction (MAD) (37). As the annulus per se is a pure fibrous structure it’s kinetic behavior can only be linked to ventricular myocardial contraction. The normal annulus is inserted firmly into the ventricular myocardium, ensuring that the ventricular contraction has appropriate effects on annular shape (2). Already three decades ago a separation between the atrial wall-MV junction and the left ventricular attachment was described in floppy MV (41). This observation was taken into consideration by Newcomb and coworkers as a possible explanation of failed repair in myxomatous degeneration: an inadequate annuloplasty might not re-fix the disjunctive annulus to the ventricle, thus the mechanical stress on leaflets and chordae was not properly corrected (42). However, the literature so far is confusing, whereby MAD is considered as the cause of MVR (41), as a constant byproduct of myxomatous MV (43), or as insignificant (44,45). It was hypothesized, that MAD could be a precursor of MVR as disjunctive areas represent weak spots vulnerable for mechanical stress (42,46). It can be speculated that MAD also could promote functional prolapse in Barlow’s syndrome. MAD could be an additional explanation for the pathological leaflet motion despite intact chordae (32,37).
The reduction of annular size by a precisely selected annuloplasty ring leads to geometrical changes allowing for a more vertical position of MV leaflets with a good zone of coaptation. The leaflets can coapt at the level of the left ventricle below the mitral annulus (14). Thus, papillary muscle traction is no longer apparent and the papillary muscles are able to descend normally to the apex of the left ventricle during systole (34). The annuloplasty ring size is an important issue with the aim to match the annulus to the increased leaflet surface. A ring sizer to cover the whole MV orifice was described to be appropriate (32). In all published series early and mid-term results are excellent. The incidence of SAM is very low despite the enlarged leaflets (14,33-36). De Paulis and associates performed a stress echo in a 4-year follow-up period showing 92% absence of any MVR, 5% trivial MVR and 3% mild MVR. No SAM was detected during stress echo (32). Although results are promising the reported number of patients is so far rather small. Nevertheless, having the entity of functional prolapse in mind and the ability of a rather simple repair at hand the number of patients diagnosed with this special form of Barlow’s syndrome might further increase.
Video (Figure 9)

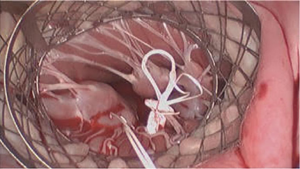
The video displays the setting of fully endoscopic approach via peri-areolar incision and groin vessel cannulation. Echo loops show the bileaflet prolapse and the paradoxical ventricular contraction leading to the functional prolapse of the posterior MV leaflet with a rather centric regurgitant jet. The distance between the papillary muscle tip and the free margin of the leaflet is measured in diastole and systole, it remains almost equal due to the paradox upward movement of the papillary muscle towards the annular plane. After aortic cross-clamping and application of cardioplegia the left atrium is cut open and the MV is visualized. Both venae cavae are snared for subsequent tricuspid valve repair (not shown). The first action is a water test presenting an almost competent valve. This is the surgical proof of concept for functional prolapse. Valve analysis shows myxomatous valve tissue and a bileaflet prolapse with chordae of normal length. Nevertheless, chordae to the posterior MV leaflet are already diseased in means of thickening and irregular structure. To achieve a posterior located coaptation zone by pulling the enlarged posterior mitral leaflet (PML) into the ventricle to avoid SAM and to prevent further degeneration with chordal elongation or even rupture and recurrent MVR artificial chordae are implanted throughout the posterior leaflet. Finally, a slightly oversized annuloplasty ring (40 mm ring after 38 mm exact measurement) is implanted. The final water test demonstrates a competent valve with a large zone of coaptation and absence of SAM which is confirmed in echo.
Conclusions
MV repair can be successfully performed in Barlow’s syndrome, minimally invasive approach and totally endoscopic techniques are safe and feasible in specialized centers. Various surgical techniques and measurements to overcome the complexity of a Barlow valve are described. Among them, the concept of “pseudo-prolapse” or “functional prolapse”, probably associated to disjunction of the MV annulus, allows for a rather simple non-resectional repair. So far, the results of this technique are promising according to literature. Further investigations are required to fully understand the various physiological and pathological movements of the MV annulus to completely understand the consequences of dilatation and disjunction.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2019.06.01). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Adams DH, Rosenhek R, Falk V. Degenerative mitral valve regurgitation: best practice revolution. Eur Heart J 2010;31:1958-66. [Crossref] [PubMed]
- Lee AP, Jin CN, Fan Y, et al. Functional Implication of Mitral Annular Disjunction in Mitral Valve Prolapse: A Quantitative Dynamic 3D Echocardiographic Study. JACC Cardiovasc Imaging 2017;10:1424-33. [Crossref] [PubMed]
- Baumgartner H, Falk V, Bax JJ, et al. 2017 ECS/EACTS guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739-91. [Crossref] [PubMed]
- Nishimura RA, Otto CM, Bonow RO, et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017;135:e1159-95. [Crossref] [PubMed]
- Carpentier AF, Adams DH, Filsoufi F. Carpentier’s reconstructive valve surgery: From valve analysis to valve reconstruction. Philadelphia: Elsevier, 2010.
- Van Praet KM, Stamm C, Sündermann SH, et al. Minimally Invasive Surgical Mitral Valve Repair: State of the Art Review. Interv Cardiol 2018;13:14-9. [PubMed]
- Svensson LG. Minimal-access “j” or “j” sternotomy for valvular, aortic, and coronary operations or reoperations. Ann Thorac Surg 1997;64:1501-3. [Crossref] [PubMed]
- Müller LC. Mini-sternotomy mitral valve surgery. In: Minimally Invasive Mitral Valve Surgery. 1st edition. In: Vohra HA, Solinas M. editors. Nova Science Publishers, Inc. 2017:145.
- Gillinov AM, Cosgrove DM. Minimally invasive mitral valve surgery: mini-sternotomy with extended transseptal approach. Semin Thorac Cardiovasc Surg 1999;11:206-11. [Crossref] [PubMed]
- Müller L, Höfer D, Holfeld J, et al. Indications and contra-indications for minimally invasive mitral valve surgery. J Vis Surg 2018;4:255. [Crossref]
- Casselman FP, Van Slycke S, Dom H, et al. Endoscopic mitral valve repair: feasible, reproducible and durable. J Thorac Cardiovasc Surg 2003;125:273-82. [Crossref] [PubMed]
- Sündermann SH, Sromicki J, Rodriguez Cetina Biefer H, et al. Mitral valve surgery: right lateral minithoracotomy or sternotomy? A systematic review and meta-analysis. J Thorac Cardiovasc Surg 2014;148:1989-1995.e4. [Crossref] [PubMed]
- Westhofen S, Conradi L, Deuse T, et al. A matched pair analysis of non-rib spreading, fully endoscopic, mini-incision technique versus conventional mini-thoracotomy for mitral valve repair. Eur J Cardiothorac Surg 2016;50:1181-7. [Crossref] [PubMed]
- Ben Zekry S, Spiegelstein D, Sternik L, et al. Simple repair approach for mitral regurgitation in Barlow disease. J Thorac Cardiovasc Surg 2015;150:1071-7.e1. [Crossref] [PubMed]
- Barlow JB, Pocock WA, Marchand P, et al. The significance of late systolic murmurs. Am Heart J 1963;66:443-52. [Crossref] [PubMed]
- Barlow JB, Bosman CK. Aneurysmal protrusion of the posterior leaflet of the mitral valve. An auscultatory-electrocardiographic syndrome. Am Heart J 1966;71:166-78. [Crossref] [PubMed]
- Bittar N, Sosa JA. The billowing mitral valve leaflet. Circulation 1968;38:763-70. [Crossref] [PubMed]
- Carpentier A, Chauvaud S, Fabiani JN, et al. Reconstructive surgery of mitral valve incompetence: ten year appraisal. J Thorac Cardiovasc Surg 1980;79:338-48. [PubMed]
- da Rocha E, Silva JG, Spampinato R, Misfeld M, et al. Barlow's Mitral Valve Disease: A Comparison of Neochordal (Loop) and Edge-To-Edge (Alfieri) Minimally Invasive Repair Techniques. Ann Thorac Surg 2015;100:2127-33; discussion 2133-5. [Crossref] [PubMed]
- Castillo JG, Anyanwu AC, El-Eshmawi A, et al. All anterior and bileaflet mitral valve prolapse are repairable in the modern era of reconstructive surgery. Eur J Cardiothorac Surg 2014;45:139-45. [Crossref] [PubMed]
- Adams DH, Anyanwu AC, Rahmanian PB, et al. Large annuloplasty rings facilitate mitral valve repair in Barlow's disease. Ann Thorac Surg 2006;82:2096-100; discussion 2101. [Crossref] [PubMed]
- Siordia JA. Current discoveries and interventions for Barlow’s disease. Curr Cardiol Rep 2016;18:73-80. [Crossref] [PubMed]
- Jouan J, Berrebi A, Chauvaud S, et al. Mitral valve reconstruction in Barlow disease: Long-term echocardiographic results and implications for surgical managements. J Thorac Cardiovasc Surg 2012;143:S17-20. [Crossref] [PubMed]
- Maisano F, Schreuder JJ, Oppizzi M, et al. The double-orifice technique as a standardized approach to treat mitral regurgitation due to severe myxomatous disease: surgical technique. Eur J Cardiothorac Surg 2000;17:201-5. [Crossref] [PubMed]
- Miura T, Ariyoshi T, Tanigawa K, et al. Technical aspects of mitral valve repair in Barlow’s valve with prolapse of both leaflets: triangular resection for excess tissue, sophisticated chordal replacement, and their combination (the restoration technique). Gen Thorac Cardiovasc Surg 2015;63:61-70. [Crossref] [PubMed]
- Fasol R, Mahdjoobian K. Repair of mitral valve billowing and prolapse (Barlow): The surgical technique. Ann Thorac Surg 2002;74:602-5. [Crossref] [PubMed]
- Perier P, Hohenberger W, Lakew F, et al. Toward a new paradigm for the reconstruction of posterior leaflet prolapse: midterm results of the "respect rather than resect" approach. Ann Thorac Surg 2008;86:718-25. [Crossref] [PubMed]
- David TE, Omran A, Armstrong S, et al. Long-term results of mitral valve repair for myxomatous disease with and without chordal replacement with expanded polytetrafluoroethylene sutures. J Thorac Cardiovasc Surg 1998;115:1279-85; discussion 1285-6. [Crossref] [PubMed]
- Borger MA, Kaeding AF, Seeburger J, et al. Minimally invasive mitral valve repair in Barlow’s disease: Early and long term results. J Thorac Cardiovasc Surg 2014;148:1379-85. [Crossref] [PubMed]
- von Oppell UO, Mohr FW. Chordal replacement for both minimally invasive and conventional mitral valve surgery using premeasured Gore-Tex loops. Ann Thorac Surg 2000;70:2166-8. [Crossref] [PubMed]
- Speziale G, Nasso G, Esposito G, et al. Results of mitral valve repair for Barlow disease (bileaflet prolapse) via right minithoracotomy versus conventional median sternotomy: A randomized trial. J Thorac Cardiovasc Surg 2011;142:77-83. [Crossref] [PubMed]
- De Paulis R, Maselli D, Salica A, et al. Mitral repair with the sole use of a semi-rigid band in a sub-population of patients with Barlow's disease: a 4-year follow-up with stress echocardiography. Interact Cardiovasc Thorac Surg 2015;21:316-21. [Crossref] [PubMed]
- Klautz RJ, Tomšič A, Palmen M, et al. Optimal surgical mitral valve repair in Barlow's disease: the concept of functional prolapse. Multimed Man Cardiothorac Surg 2016; [Crossref] [PubMed]
- Lawrie GM. Barlow disease: simple and complex. J Thorac Cardiovasc Surg 2015;150:1078-81. [Crossref] [PubMed]
- Lawrie GM, Earle EA, Earle NR. Nonresectional repair of Barlow mitral valve: Importance of dynamic annular evaluation. Ann thorac Surg 2009;88:1191-6. [Crossref] [PubMed]
- Tomšic A, Hiemstra YL, Bissessar DD, et al. Mitral valve repair in Barlow's disease with bileaflet prolapse: the effect of annular stabilization on functional mitral valve leaflet prolapse. Interact Cardiovasc Thorac Surg 2018;26:559-65. [Crossref] [PubMed]
- Enriquez-Sarano M. Mitral Annular Disjunction: The Forgotten Component of Myxomatous Mitral Valve Disease. JACC Cardiovasc Imaging 2017;10:1434-6. [Crossref] [PubMed]
- Sanfilippo AJ, Harrigan P, Popovic AD, et al. Papillary muscle traction in mitral valve prolapse: quantitation by two-dimensional echocardiography. J Am Coll Cardiol 1992;19:564-71. [Crossref] [PubMed]
- David D, Michelson EL, Naito M, et al. Diastolic "locking" of the mitral valve: the importance of atrial systole and intraventricular volume. Circulation 1983;67:640-5. [Crossref] [PubMed]
- Ehlers KH, Engle MA, Levin AR, et al. Left ventricular abnormality with late mitral insufficiency and abnormal electrocardiogram. Am J Cardiol 1970;26:333-40. [Crossref] [PubMed]
- Hutchins GM, Moore GW, Skoog DK. The association of floppy mitral valve with disjunction of the mitral annulus fibrosus. N Engl J Med 1986;314:535-40. [Crossref] [PubMed]
- Newcomb AE, David TE, Lad VS, et al. Mitral valve repair for advanced myxomatous degeneration with posterior displacement of the mitral annulus. J Thorac Cardiovasc Surg 2008;136:1503-9. [Crossref] [PubMed]
- Eriksson MJ, Bitkover CY, Omran AS, et al. Mitral annular disjunction in advanced myxomatous mitral valve disease: echocardiographic detection and surgical correction. J Am Soc Echocardiogr 2005;18:1014-22. [Crossref] [PubMed]
- Angelini A, Ho SY, Anderson RH, et al. A histological study of the atrioventricular junction in hearts with normal and prolapsed leaflets of the mitral valve. Br Heart J 1988;59:712-6. [Crossref] [PubMed]
- Angelini A, Ho SY, Anderson RH, et al. Disjunction of the mitral annulus in floppy mitral valve. New Engl J Med 1988;318:188-9. [Crossref] [PubMed]
- Dejgaard LA, Skjolsvik ET, Lie OH, et al. The mitral annulus disjunction arrhythmic syndrome. J Am Coll Cardiol 2018;72:1600-9. [Crossref] [PubMed]
- Hoefer D, Mueller L. Setup for totally endoscopic mitral valve surgery, diagnosis and repair of a functional prolapse in Barlow’s syndrome, and repair results. Asvide 2019;6:183. Available online: http://www.asvide.com/article/view/32474
Cite this article as: Hoefer D, Mueller L. Minimally invasive mitral valve repair for Barlow’s syndrome with functional prolapse using artificial chords. J Vis Surg 2019;5:60.