Robotic pulmonary metastasectomy using precision multimodal surgical navigation
Introduction
In the past decade, robot-assisted thoracic surgery has become more popular in the domain of pulmonary resections (lobectomies and segmentectomies). 3D high definition vision, tremor filtering, and the 7 degrees of movement help the surgeon perform very precise and anatomical dissections indeed (1,2). Nevertheless, localizing the target lesion(s) notably in the case of lung metastases without actually palpating the lung, might prove to be a very difficult task.
Nowadays, we live in an interesting era where the development of such an approach is witnessing new technical leaps; the integration of 3D models peroperatively, and the ability to inject indocyanine green to visualize the lesion as well as the intersegmental plane.
In the article, we review the different strategies that we employ in our daily practice in order to optimize our chances of finding the target lesions, and performing effective resections with acceptable margins.
Lung metastectomy, where do we come from? Where are we today?
Discriminated malignant diseases in the lung were always considered to be an ominous sign by clinicians, and were associated with very poor prognosis. Despite Barney’s report on a twelve-year cure following nephrectomy for adenocarcinoma and lobectomy for solitary metastasis back in 1945 (3), lack of big data and solid scientific evidence fueled the debate of long-term benefits behind this kind of surgery.
It was not until Pastorino et al. (4) published the results of 5,206 cases of lung metastectomy from the international registry of lung metastases in 1997, that evidence about the safety and potentiality of curing metastatic diseases started to surface.
In regards to the surgical technique, traditional teaching insists on the importance of bimanual palpation of the ipsilateral lung to detect and resect all possible nodules. As video-assisted thoracoscopic surgery (VATS) became the recommended approach for the treatment of early stage lung cancer, more surgeons started using it for the treatment of lung metastases, thus imaging and peroperative inspection started to replace the role of bimanual palpation as a detection method. The only withdraw is the inability to detect non-imaged small nodules on CT scans, and deep nodules when it comes to direct inspection by VATS. It’s true that this idea is generally supported by reports in the literature (5), but let’s not forget that these reports go back to an era when a fine cut CT scan consisted of 5 mm slices, in contrast to today’s imaging machines’ generation where obtaining 0.5 mm slices is possible, and that pure ground glass nodules are detectable on CT scans but hardly ever palpable during open surgery.
An interesting prospective work is that of Eckardt et al. (6) published in 2012, where patients underwent VATS metastectomy by one surgical team, followed by immediate thoracotomy with bimanual palpation and resection of all palpable nodules by a second surgical team during the same anesthesia. Interestingly, both teams observed the same number of suspicious lesions on the preoperative CT scans. Bimanual palpation found 29 more nodules than VATS, out of those 21% (n=6) were metastasis and one was primitive lung cancer. However, despite being a relatively recent work, one must note that again, the slice thickness was of 3–5 mm.
PET scan can be a valuable tool to detect lung metastases with a sensitivity reaching up to 67.5% as shown by Fortes et al. (7). Size could matter, notably in case of melanoma as sensitivity rises from 7.9% for lesions of 4 to 5 mm to 100% in lesion that are 12 mm or bigger in size (8). Type of primary cancer seems to be an important factor too, as some studies showed that spiral CT scan might be superior than PET in detecting secondary metastases from malignant bone tumors (9).
The bottom-line is that VATS remains less invasive than thoracotomy, causing less pain, morbidity and immunological response, and helps to preserve a good quality of life allowing subsequent adjuvant treatment. So the question to answer is the following: Is manual palpation of the lung necessary in patients undergoing pulmonary metastasectomy? (10). So far, not enough data is available on the impact of those nodules on the overall survival of the patients (11), and that sequential thoracic resections when these nodules become detectable is feasible and prolong survival in selected patients using a video-assisted approach.
Our multimodal system
After approving the surgical indication in a specialized multidisciplinary meeting, all patients with suspected lung metastases are evaluated in the same manner as those who undergo lung resections for primitive lung cancer.
In all cases, we obtain a recent (not more than 1 month old) high definition chest CT scan with infra millimetric slices (usually 0.5–0.6 mm), and a PET scan.
In the case of the peripheral nodule that retracts the visceral pleura, we might solely depend on the peroperative direct vision to detect it, and carry out a wedge resection, those cases are generally reserved for VATS.
In the daily practice, we know that deeper nodules from lung surface, have less chances of being detected by direct vision. This is where pleural dye marking using radial endobronchial ultrasound and virtual bronchoscopy becomes of a great benefit. The procedure is performed minutes before surgery by an experienced pulmonologist, it consists of directing the bronchoscope into smallest bronchus following a route predefined by uploading the CT scan into a virtual bronchoscopy program (LungPoint® Planner, Broncus Medical Inc., San Jose, CA, USA). Upon reaching the most distal bronchus, the guide sheath with the r-EBUS probe is inserted into the working channel and pushed towards the lesion in order to reach the subpleural space. The probe was then removed and 1 mL of methylene blue (5 mg/1 mL) was injected and rinsed with 20 mL of air.
Lachkar et al. published our institutional technique, the dye was visible in all patients, and the same operative precision was judged impossible in 21 out of the 24 published cases by the operating surgeon (12). We recently started testing endobronchial injection of indocyanine green as shown in our video (Figure 1).

When nodules are more central, we send the anonymized CT scan to a private company ‘Visible Patient™’ in Strasbourg, France, in order to create 3D models. This process might take up to 5 days. Those models help us decide on the operative strategy (sublobar vs. lobar resections), visualize the resections’ margins, the intersegmental planes, and study beforehand the personal anatomy of the patients. We have been using and developing this mode of planning for all segmentectomies; for primitive and metastatic diseases since 2016 (14,15).
Those 3D models could be visualized on any PC, portable cellphone, and tablet. The other advantage is that they can be integrated into the robotic console allowing a virtual reality-like experience during surgery.
Where are we heading?
Patients with lung metastases are at risk of having future recurrence. That’s why we highly recommend minimally invasive approached if an R0 resection is possible, in order to keep a good quality of life, whilst being able to achieve further resections in the future if needed. Adhesions after VATS and thoracotomy are not really comparable.
In the video, we show our perioperative planning and operative strategy in the case of a 57-year-old female patient who was operated of a thyroid cancer, and received radioactive iodine postoperatively. Nevertheless, she had persistent high level of thyroglobulin, On the follow-up CT scan there was and 3 suspected lesions. PET scan showed hypermetabolism of one of the left lower lobe nodules. This is the first surgery (left robot-assisted S9+10), the patient had a second lung metastectomy of the right side one month after the first operation (Figure 1).
In terms of peroperative nodules’ detection, the first-in-human demonstration of identifying pulmonary nodules near-infrared (NIR) imaging using indocyanine green without previous knowledge of their location or existence was reported by OT Okusanya et al. in 2014, this showed that NIR can detect poorly visualized lesions on high quality CT that are non-palpable during thoracotomy (16). NIR enhances the number of detected nodules compared to direct vision during VATS surgery, in a series of 34 patients, it led to the detection of 9 more nodules, out of which 4 were confirmed malignant (17).
A possible future NorthStar could be the development of peroperative molecular imaging, a technique that is still in phase I trials currently, and could improve lung nodules’ detection (18). One limitation of both techniques is the nodule depth from the lung surface.
Our results
Between 2012 and 2018, we operated 168 patients of suspected pulmonary metastectomy, 161 of them by minimally invasive approaches (RATS and VATS). Twenty-four of which (14.9%) were re-operated for contralateral metastases or ipsilateral recurrence over the same period of time.
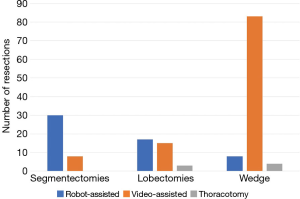
Of those operated by VATS the majority had wedge resections. In our practice, we prefer using a robot-assisted technique notably when it comes to segmentectomy, the robot helps us achieve precise dissections, and visualize the intersegmental plane by using indocyanine green.
Only 3 lobectomies and 4 multiple wedges resections were performed by thoracotomy (Figure 2).
In terms of lymphadenectomy, we do selective lymph nodes dissections in the case of lobectomies and segmentectomies only.
The number of patients operated by RATS for lung metastases was (n=55) (34 males, and 20 females; 1 bilaterally). All of the aforementioned population had previous history of cancer median age was 65 years. Surgical resections were in the forms of single wedge (n=5), multiple wedges (n=3), segmentectomies (n=30), and lobectomies (n=17). Pleural dye marking alone was used in 3 wedge resections. We started having access to 3D lung modeling in 2017, this technique was used in 11 out of the 15 segmentectomies we operated since then. Four of which had combined pleural dye marking in order to have good margins mainly because the target lesion was close to the intersegmental plane.
Mean duration of stay is 5.3 days. Rate of surgical complications was 20.7% as follows: seven grade I, three grade II, and one grade III (recurrent nerve paralysis) complications. In other words, 90.9% of the complications are classed under grade I and II according to Dindo’s classification.
In 54 patients, resection margins were R0. The only incomplete resection occurred in the case of segmentectomy for renal cell carcinoma where the bronchial limits were R1.
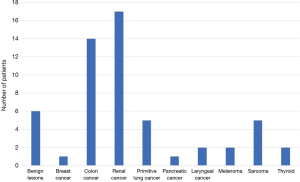
Final histopathological analysis of the lesions showed benign lesions (n=6), primitive lung cancer (n=5), breast cancer (n=1), colon cancer (n=14), renal cancers (kidney, testicular and bladder) (n=17), laryngeal cancer (n=2), melanoma (n=2), sarcoma (n=5), pancreatic cancer (n=1), thyroid cancer (n=2) (Figure 3).
It’s noteworthy to say that 9% of the patients we thought having secondary lung metastases, turned out to have a primitive lung cancer, and almost 11% to have benign lesions. This in fact, changes the whole management strategy, and could be an additional argument for going for surgery.
Conclusions
Lung metastectomy by robot-assisted thoracic surgical approach is feasible and safe, the robot’s freedom of movement and high definition may facilitate the surgeon’s mission notably when it comes to sublobar resections. In our opinion, preoperative planning and multimodal navigation (3D models’ integration into the robot, endobronchial methylene blue, and indocyanine green) remain the key behind a successful R0 surgery specially for deep lesions necessitating segmentectomy. One must keep in mind that complete resection is the oncologic cornerstone of this kind of surgery, thus we need not to hesitate if thoracotomy is required to achieve that.
Acknowledgments
We highly acknowledge the contribution of our database manager, Mr. Mbadinga Frankie, without whom, this work would have been more difficult to achieve. We also express our gratitude to Dr. Raghda Majeed for her help in proof reading this article.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Michel Gonzalez) for the series “Advancement in the Surgical Treatment of Pulmonary Metastasis” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2019.05.03). The series “Advancement in the Surgical Treatment of Pulmonary Metastasis” was commissioned by the editorial office without any funding or sponsorship. JMB serves as an unpaid editorial board member of Journal of Visualized Surgery from Dec 2017 to Nov 2019 and reports personal fees from Intuiitve Surgery, during the conduct of the study. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- O'Sullivan KE, Kreaden US, Hebert AE, et al. A systematic review and meta-analysis of robotic versus open and video-assisted thoracoscopic surgery approaches for lobectomy. Interact Cardiovasc Thorac Surg 2019;28:526-34. [Crossref] [PubMed]
- Veronesi G. Robotic lobectomy and segmentectomy for lung cancer: results and operating technique. J Thorac Dis 2015;7:S122-30. [PubMed]
- Barney JJ. A twelve-year cure following nephrectomy for adenocarcinoma and lobectomy for solitary metastasis. Trans Am Assoc Genitourin Surg 1945;37:189-91. [PubMed]
- Pastorino U, Buyse M, Friedel G, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 1997;113:37-49. [Crossref] [PubMed]
- Parsons AM, Detterbeck FC, Parker LA. Accuracy of helical CT in the detection of pulmonary metastases: is intraoperative palpation still necessary?. Ann Thorac Surg 2004;78:1910-6; discussion 1916-8.
- Eckardt J, Licht PB. Thoracoscopic versus open pulmonary metastasectomy: a prospective, sequentially controlled study. Chest 2012;142:1598-602. [Crossref] [PubMed]
- Fortes DL, Allen MS, Lowe VJ, et al. The sensitivity of 18F-fluorodeoxyglucose positron emission tomography in the evaluation of metastatic pulmonary nodules. Eur J Cardiothorac Surg 2008;34:1223-7. [Crossref] [PubMed]
- Mayerhoefer ME, Prosch H, Herold CJ, et al. Assessment of pulmonary melanoma metastases with 18F-FDG PET/CT: which PET-negative patients require additional tests for definitive staging? Eur Radiol 2012;22:2451-7. [Crossref] [PubMed]
- Franzius C, Daldrup-Link HE, Sciuk J, et al. FDG-PET for detection of pulmonary metastases from malignant primary bone tumors: comparison with spiral CT. Ann Oncol 2001;12:479-86. [Crossref] [PubMed]
- Macherey S, Doerr F, Heldwein M, et al. Is manual palpation of the lung necessary in patients undergoing pulmonary metastasectomy? Interact Cardiovasc Thorac Surg 2016;22:351-9. [Crossref] [PubMed]
- Jaklitsch MT, Mery CM, Lukanich JM, et al. Sequential thoracic metastasectomy prolongs survival by re-establishing local control within the chest. J Thorac Cardiovasc Surg 2001;121:657-67. [Crossref] [PubMed]
- Lachkar S, Baste JM, Thiberville L, et al. Pleural Dye Marking Using Radial Endobronchial Ultrasound and Virtual Bronchoscopy before Sublobar Pulmonary Resection for Small Peripheral Nodules. Respiration 2018;95:354-61. [Crossref] [PubMed]
- Sarsam M, Peillon C, Baste JM. Robot-assisted left lateral & posterior basal segmentectomy (S9+10) with multimodal navigation. Asvide 2019;6:152. Available online: http://www.asvide.com/article/view/32099
- Baste JM, Soldea V, Lachkar S, et al. Development of a precision multimodal surgical navigation system for lung robotic segmentectomy. J Thorac Dis 2018;10:S1195-204. [Crossref] [PubMed]
- Le Moal J, Peillon C, Dacher JN, et al. Three-dimensional computed tomography reconstruction for operative planning in robotic segmentectomy: a pilot study. J Thorac Dis 2018;10:196-201. [Crossref] [PubMed]
- Okusanya OT, Holt D, Heitjan D, et al. Intraoperative near-infrared imaging can identify pulmonary nodules. Ann Thorac Surg 2014;98:1223-30. [Crossref] [PubMed]
- Mao Y, Chi C, Yang F, et al. The identification of sub-centimetre nodules by near-infrared fluorescence thoracoscopic systems in pulmonary resection surgeries. Eur J Cardiothorac Surg 2017;52:1190-6. [Crossref] [PubMed]
- Predina JD, Fedor D, Newton AD, et al. Intraoperative Molecular Imaging: The Surgical Oncologist's North Star. Ann Surg 2017;266:e42-4. [Crossref] [PubMed]
Cite this article as: Sarsam M, Peillon C, Baste JM. Robotic pulmonary metastasectomy using precision multimodal surgical navigation. J Vis Surg 2019;5:53.