
Image-guided pulmonary metastasectomy in the hybrid operating room
Introduction
An increasing trend in metastasectomy across cancer types and anatomic sites has been observed over the past decade; this could attribute to the advance in chemotherapy, radiotherapy and surgical techniques (1). Pulmonary metastasectomy (PME) specifically represents the second fastest growing type of metastasectomy after liver metastasectomy (1,2). The oncologic principles of pulmonary metastasectomy (PME) include (3,4):
- The primary tumor must be under control;
- No non-controllable extrathoracic metastases exist;
- There is adequate pulmonary reserve such that all pulmonary metastases can be completely resected;
- There is no better medical treatment for further cancer volume reduction.
Based on 5,206 patients enrolled in Europe and North America, the landmark publication from the International Registry of Lung Metastases reported that despite widely varied survival rates across different cancer histologies, significantly higher long-term survival was noted in patients who had complete removal of metastatic lesions (5). These findings influenced the strategic approach to PME.
To identify and resect an ill-defined pulmonary nodule with accuracy relies on a surgeon’s ability to interpret and integrate preoperative images with intraoperative findings. In the 1990s, Suzuki et al. proposed that if a target lesion’s diameter was smaller than 10mm or if the distance from the visceral pleura was greater than 5 mm, the risk of being undetectable during thoracoscopic surgery would reach 63% (6). The size, depth, and proportion of solid component of the pulmonary nodule are key factors affecting detection during thoracoscopic resection regardless of tumor biology (7). Nakashima et al. retrospectively evaluated 224 pulmonary metastatic nodules of different cancer origins that underwent thoracoscopic PME and proposed that preoperative localization should be strongly considered if at least two of the following criteria are met (8):
- The nodule is less than 5 mm in maximum diameter;
- The ratio of the maximum nodular diameter to its depth from the visceral pleural surface is less than 0.5;
- The nodule presented with low density image on CT.
Broadly, nodule localization involves introducing fiducial markers into the lung parenchyma to facilitate detection and guide resection of pulmonary nodules during minimally invasive thoracoscopic surgery. Fiducial markers include metal (e.g., hook-wire and micro-coil) (9-11), dye (e.g., patent blue V dye) (12), radiopaque agents (e.g., lipiodol) (13-15), fluorescent contrast (e.g., indocyanine green) (16,17), and radiolabeled agents (e.g., 99mTc-MAA) (18-20). The clinical demand for radical PME has driven advances in localization techniques to assist surgeons in localizing pulmonary lesions during minimally invasive procedures.
At Toronto General Hospital (Toronto, ON, Canada), a hybrid operating room (HOR) named the Guided Therapeutics operating room (GTx OR) was constructed with the goal of augmenting surgical performance through application of state-of-the-art imaging technologies (21). This includes a multi-detector computed tomography (MDCT) (Somatom Definition Flash, Siemens Healthcare GmbH, Erlangen, Germany) platform and a multi-axis robotic C-arm cone-beam CT (CBCT) (Artis Zeego, Siemens healthcare GmbH, Erlangen, Germany) which are positioned to provide on-site radiological intervention and real-time intraoperative image guidance for localization-guided thoracoscopic surgery, i.e., image-guided video-assisted thoracoscopic surgery (iVATS) (Figure 1) (21,22).
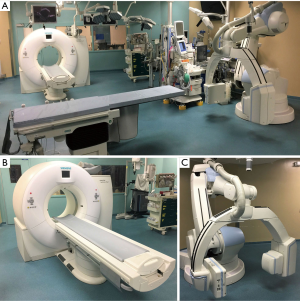
In the following sections, current practices for localization-guided thoracoscopic resection relevant to PME are reviewed, within the context of multimodality HORs.
Pulmonary metastases: diagnosis and resection challenges
Pulmonary metastases develop in up to 54% of patients diagnosed with extra-thoracic malignancy (either on initial staging or over the course of disease progression) (23). Based on retrospective data in patients with previously confirmed extra-thoracic cancer, Caparica et al. reported that metastatic disease account for 64% of newly identified pulmonary nodules; the remainder were diagnosed as primary lung cancers (26.3%) or benign lesions (9.6%) (24). Although certain features on imaging such as multiple nodules (24,25), cavitation, or necrosis (24,26) are suggestive of a metastatic etiology, the differential diagnosis often remains broad given the variety of pulmonary benign and malignant lesions (27). As the treatment plan is shaped significantly by the underlying pathology, further intervention for tissue diagnosis is therefore inevitable (e.g., bronchoscopy, transthoracic needle biopsy, or surgical biopsy). Meta-analyses on transbronchial and CT-guided transthoracic biopsy in current practice report diagnostic yields of 70% and 92.1%, respectively; however, this is often influenced by the size and location of the target lesion(s) (28-30). The advance in imaging has enabled early detection of newly developed pulmonary nodules, even when less than 1cm in maximum diameter, in patients with extra-thoracic malignancy (31). Inherent limitations with transthoracic/transbronchial sampling make surgical biopsy irreplaceable for precise diagnosis in select patients.
Surgical radicality and a long disease-free interval are positive prognostic factors for patients undergoing pulmonary metastasectomy (3,32). Welter et al. reported that pulmonary metastases with certain aggressive growth patterns on pathological evaluation, such as interstitial growth and pleural infiltration, are significantly correlated with local recurrence (33). The authors suggested to remove aggressive-grown pulmonary metastatic nodules with at least 7mm rim of healthy tissue as the safety margins (33). Since the goals of complete resection and maintaining adequate pulmonary reserve are sometimes in conflict, removal of metastatic lesions with precision could be challenging for surgeons, particularly in the era of minimally invasive surgery (8).
Literature review reveals few if any studies on pulmonary nodule localization techniques in HORs specifically for patients with known or presumed pulmonary metastases (34). Nevertheless, the challenges for localizing small and deep-located pulmonary nodules for thoracoscopic resection are shared for both primary and secondary lung malignancies, both with regards to accurate tissue diagnosis and achieving complete surgical resection.
One-stage workflow for localization-guided pulmonary resection in the hybrid operating room
Typically, a two-stage workflow is required to resect a small or deeply-located pulmonary nodule: (I) preoperative CT-guided transthoracic implantation of a fiducial marker in the interventional CT suite followed by (II) patient transfer to the operating room for surgical resection. The need for patient transfer and OR preparation in traditional two-stage localization-guided resection exposes risks from procedure-related adverse events (e.g., pneumothorax, parenchymal hematoma) or from dislodgement of fiducial markers. Multimodal HORs, integrating imaging systems into an operating room, have the resources on-site to allow localization and resection to be performed as a single unified procedure, offering the potential to reduce total procedure time, allow immediate management of any complications, and possibly increase the success rate of localization-guided PME (35,36).
There is limited literature comparing the safety and efficacy between preoperative and intraoperative localization procedures. Chao et al. compared preoperative MDCT-guided versus intraoperative C-arm CBCT-guided localization-guided thoracoscopic resections in consecutive patients with solitary pulmonary nodules (36). There was no significant intergroup difference in patient demographics and nodular characteristics. The localization procedural time and successful intraoperative nodule localization were also statistically comparable in both groups. However, patients undergoing preoperative localization had significant longer interval times between localization and surgery, higher risk of pneumothorax, but shorter global OR utilization time (36).
Additional radiation exposure for patients and OR staff is an important safety concern to consider when introducing CBCT for image-guided operations. Since radiation exposure may vary with the frequency of CBCT acquisitions, patients who undergo iVATS require adequate radiation exposure monitoring. One study evaluated this risk by placing thermoluminescent dosimeters (TLDs) (UD-802A; Panasonic, Osaka, Japan) on patients’ chest walls around the marking field to quantify the radiation exposure with preoperative versus intraoperative localization. The authors found that patients who undergo localization with preoperative CT-guidance had statistically similar radiation exposure compared with intraoperative CBCT-guidance (mean 6.88 vs. 3.65 mSv, P=0.51) (36). For context, the effective dose of a conventional diagnostic chest CT is approximately 6.5 mSv (37). Conversely, Chen et al. reported significantly higher radiation exposure in patients undergoing intraoperative CBCT-guided localization versus preoperative CT-guided localization. This may have been related to the relative lack of experience with CBCT in this study (38).
Results from these studies indicate that marker-guided thoracoscopic resection under the guidance of intraoperative CBCT is a safe and efficient alternative over conventional two-stage localization/resection, notwithstanding several debates: (I) longer global OR time with iVATS introduces economic and administrative challenges that must be compared with the benefits of reduced preoperative CT use (36,39), (II) radiation exposure for localization in HORs remains an unresolved safety concern (38), and (III) the sensitivity of these imaging modalities to patient positioning could increase procedure time and radiation exposure in centers less experienced with HOR use (40).
Imaging systems in the hybrid operating room
Several different imaging systems for image acquisition and guidance for localization in HORs have been described in the literature.
Multi-detector CT system
Fumimoto et al. described the intraoperative MDCT-guided localization approach (41). Following induction of general anesthesia, the patient is first placed in supine or prone position (depending on the location of the target lesion) on a free-floating table that is compatible with the gantry of the HOR MDCT (Somatom Definition AS+SLID, Siemens healthcare GmbH, Erlangen, Germany). After completion of the placement of the fiducial material under MDCT-guidance, the patient is repositioned into lateral decubitus position for thoracoscopic resection; meanwhile, the images acquired by MDCT are processed to generate 3D volumetric imaging of the pulmonary lesion and radio-opaque marker. The authors demonstrated the feasibility of image-guided thoracoscopic resection based on the combination of the 3D reconstructed imaging and C-arm fluoroscopy in terms of accuracy and efficiency. Although localization and resection are performed in one-stage, the patient repositioning could still be associated with risks from fiducial dislodgement or gradual onset of procedural complications (41).
The O-arm mobile 2D/3D imaging system
Ohtaka et al. demonstrated intraoperative O-arm mobile CBCT-guided thoracoscopic resection (O-arm; Metronic Japan Co., Ltd., Tokyo, Japan) (Figure 2) (42). After induction of general anesthesia, the patient is placed in lateral decubitus position on an operating table that fits in the gantry of the O-arm imaging system. The visceral pleura adjacent to the target lesion is stained with indigo carmine dye (Daiichi Sankyo Co., Ltd., Tokyo, Japan) using the intrathoracic stamping method followed by marking with a needle with 4-0 polydiaxonone thread (43). Thoracoscopic resection of the target is performed based on its positional relationship with the needle defined by the 3D configuration generated from intraoperative O-arm images. Although the authors demonstrated 90% success rate of thoracoscopic resection with this approach, the primary technical challenge was related to the limited field of view of the O-arm (20 cm in diameter, 15 cm in length in this series). As a result, more than half of patients in this series required repeated O-arm image acquisitions to optimize imaging, which potentially lead to extra procedural time and radiation exposure (42).
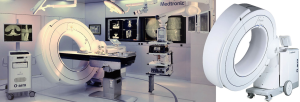
The C-arm cone-beam computed tomography system
The first clinical series of C-arm CBCT-guided localization-based thoracoscopic resection for pulmonary nodules in a HOR was performed at Brigham & Women’s Hospital (Boston, MA, USA) (22). In the HOR, the patient is positioned in lateral decubitus after induction of general anesthesia. The C-arm CBCT (Artis Zeego, Siemens healthcare GmbH, Erlangen, Germany), a robotic multi-axis C-arm fluoroscopy imaging system, permits intraoperative image acquisition for 3D reconstruction (44). When a pulmonary nodule is registered in the 3D-CBCT image dataset, the localization access path to the target can be generated by needle guidance software (Syngo iGuide; Siemens healthcare GmbH, Erlangen, Germany); this allows accurate percutaneous insertion of the introducer needle and delivery of fiducial materials under fluoroscopic guidance (Figure 3). The C-arm CBCT can be used for intraoperative fluoroscopy, allowing immediate commencement of thoracoscopic resection without the need for patient or equipment repositioning (22). However, C-arm CBCT requires a 200-degree rotation for image acquisitions and the cylindrical field of view is smaller than MDCT (24 cm in diameter, 18.5 cm in length vs. 50 cm in diameter, 200 cm in maximum length). These limitations make careful attention to patient and C-arm CBCT positioning to ensure optimal image quality while avoiding accidental collisions (40).

Marking techniques used in the hybrid operating room
Marking procedures consist of transthoracic insertion of an introducer needle followed by delivery of fiducial materials under image guidance in the HOR. The specific type of fiducial material used is not inherently linked to the decision to use MDCT, O-arm CBCT, or C-arm CBCT. Fiducial material-specific localization techniques as well as the utilization of image guidance are reviewed here (Table 1).
Table 1
| Technique | Limitations | Advantages in the HOR setting |
|---|---|---|
| Transthoracic administration | ||
| Overview | Two-staged workflow; visceral pleural injury related complications; potentially unsuitable for repeated procedures; difficult to approach apical, mediastinal, and basal regions | Single-staged workflow; intraoperative image acquisition and 3D reconstructed; real-time image guidance |
| Hook-wire/micro-coil | Risk of dislodgement: hook-wire > micro-coil; chest wall pain: hook-wire >micro-coil; risk of systemic embolism, air or fiducial | Shortened interval time at risk |
| Colored dye | Intra-alveolar diffusion or pleural spillage | Shortened interval time at risk |
| Lipiodol | Risk of migration and systemic embolism | Dot-shaped enhancement, advantageous for 3D configuration |
| 99mTC-MAA | Intra-alveolar diffusion or pleural spillage; additional time for scintigraphy confirmation; additional gamma probe; 6.02-h half-life of 99mTc | Fluoroscopy-confirmation by administrating 99mTc-MAA mixed with iodinated contrast |
| Transbronchial administration | ||
| ENB | Lack of real-time imaging of target lesions | Real-time augmented fluoroscopy guidance |
| Systemic administration | ||
| Indocyanine Green | Limited tissue penetration for NIR fluorescence; non-selective accumulation of ICG; no additional localization procedures required | |
HOR, hybrid operating room; ENB, electromagnetic navigated bronchoscopy; ICG, indocyanine green; IV, intravenous.
Hook-wire or micro-coil
The hook-wire (DuaLok; Bard Peripheral Vascular, Inc., Tempe, AZ, USA) is delivered through an introducer needle to hook in lung parenchyma adjacent to the target lesion (35,46). The wire-tail extending out through the chest wall is cut short and covered with sterile gauze pad prior to surgical resection. The micro-coil (Vortx-18, Diamond Shape, Boston Scientific, Marlborough, MA, USA) deployed similarly, but is instead completely embedded within the parenchyma adjacent to the target lesion (16,21). Once either a hook-wire or micro-coil is placed, the markers are localized by intraoperative fluoroscopy for thoracoscopic resection.
Yu et al. reported 100% success rate of C-arm CBCT-guided hook-wire localization followed by thoracoscopic resection (Table 2). Neither hook-wire dislodgment nor procedural pneumothorax requiring extrapleural drainage were noted (46).
Table 2
| Fiducials | Authors | Year | Procedures (N) | Size (mm)1 | Pleural depth (mm)1 | Localization time (min) | Success rate2 | Reasons of failure |
|---|---|---|---|---|---|---|---|---|
| MDCT-guided approach | ||||||||
| Lipiodol | Fumimoto |
2018 ( |
32 | 10.7 | 18.0 | 15.8 | 100% | |
| ICG + micro-coil | Ujiie |
2017 ( |
20 | 12 | 14 | 35 | 90% (18/20) | 2 ICG-labeled nodules were undetectable |
| O-arm CBCT-guided approach | ||||||||
| Indigo carmine dye | Ohtaka |
2014 ( |
10 | 10 | N/A | N/A | 90% (9/10) | 1 lesion couldn’t be seen by the O-arm CT |
| C-arm CBCT-guided approach | ||||||||
| Metallic T-bar | Gill |
2015 ( |
23 | 13 | N/A | 39.4 | 87% (20/23) | 3 fiducial dislodgments |
| Hook-wire | Yu |
2018 ( |
32 | 9.1 | 10.8 | N/A | 100% | |
| PBV | Yang |
2016 ( |
25 | 10 | 7 | 46 | 92% (23/25) | Procedural complications |
| Hook-wire/PBV/ICG | Chao |
2018 ( |
100 | 7.94 | 10.00 | 20.58 | 94% (94/100) | 2 procedural complications; 4 fiducial dislodgments |
| ENB for biopsy | Pritchett |
2018 ( |
92 | 16.0 | N/A | N/A | 83.7% (77/92)3 | |
1, mean size/depth from the visceral pleura; 2, successful marker-guided resection; 3, diagnostic yield equals to the number of malignant and benign lesions diagnosed (n= 63 + 14) divided by total number of lesions (n=92). MDCT, multi-detector computed tomography; O-arm, O-arm mobile computed tomography; CBCT, cone-beam computed tomography; ENB, electromagnetic navigation bronchoscopy; PBV, patent blue V dye; ICG, Indocyanine green.
The comparison of hook-wire and micro-coil localization systems was performed in the setting of preoperative MDCT-guidance by Hwang et al. Successful marker-guided resection rates, localization time, and complication rates were similar. However, a significantly higher dislodgement rate and pain score was seen in the hook-wire group (49). These differences were probably attributed to the additional tension associated with the anchoring of the wire tail between the chest wall and lung parenchyma (49).
The limitation of hook-wire/micro-coil-guided localization is its potential for dislodged during patient transport and complications associated with visceral pleural injury (11,50); both drawbacks could potentially be reduced in the setting of HORs (36,46). Though only seen in a small number of case reports, percutaneous insertion of metallic material into the lung parenchyma may be associated with life-threatening systemic embolism via accidental introduction of air during the procedure (i.e., air embolism) or of the fiducial material itself (50-53).
Colored dye
Given the discomfort and risks associated with insertion of metallic fiducials into the lung parenchyma, several authors have explored using markers that can be broken down and excreted by the body; one example is colored dyes, e.g., methylene blue. During the operation, the dye-labeled targets can be identified by standard white light thoracoscopic image without additional imaging modalities. The efficacy of most colored dye markers is limited by their inherent tendency for rapid diffusion within parenchyma or spillage into the pleural cavity (54). PBV dye (patent blue V 2.5%, Guerbet, Villepinte, France) is notable for its relatively lower diffusion, and thus has been often selected as a more ideal marker for localization of pulmonary nodules (47,55).
Yang et al. reported 92% (23/25) successful C-arm CBCT-guided PBV dye-guided resection in the HOR (Table 2). Two localization failures occurred but were attributed to the percutaneous approach rather than the dye itself. A procedural pneumothorax that prevented repeat percutaneous localization in one patient; accidental transdiaphragmatic puncture of the liver occurred in the other patient (47).
Localization with PBV dye is associated with several drawbacks: (I) despite its advantages over other dyes there is nonetheless some amount of diffusion after administration (55), (II) it is less effective when marking targets that are either deeply-located or in anthracotic/pigmented lung tissue (55), (III) sporadic anaphylactic shock has been reported in patients receiving PBV dye for lymphatic mapping and pulmonary nodule localization (56,57).
Lipiodol
To address the disadvantages of colored dye, CT contrast is introduced for localization. Since barium injection could elicit local inflammation which may lead to misinterpretation on pathology, locally-injected lipiodol is often preferred (13-15). In this approach, 0.2–0.5 mL of lipiodol (Lipiodol Ultra Fluid; Guerbet, Villepinte, France) is injected into the lung parenchyma under MDCT guidance (15,41). C-arm fluoroscopy is then used to identify the radiopaque marking to guide thoracoscopic resection.
Fumimoto et al. reported 100% successful MDCT-guided lipiodol localization followed by fluoroscopy-guided resection in the HOR (Table 2). All lipiodol-labeled pulmonary nodules were detected by intraoperative fluoroscopy as clear spots without diffusion or migration (41).
The hydrophobic properties of lipiodol present a potential risk for embolism in the event of accidental injection into the bloodstream (14); additionally, a case of postoperative pneumonitis secondary to endobronchial migration of lipiodol from contralateral marking has been reported (58). When administering lipiodol, precautions must be taken to avoid endo-bronchial or intravascular injection by applying gentle negative pressure via the syringe before injection and to inject only a small volume (15).
Indocyanine green
The least desirable feature of colored dye-guided thoracoscopic resection is that the efficacy is compromised by pigmentation of surrounding lung tissue. Indocyanine green (ICG; Lake Forest, IL, USA) is a near-infrared (NIR) fluorophore that can absorb and emit NIR light at a peak wavelength of around 790 and 830 nm, respectively (16,59). Low tissue autofluorescence and limited tissue absorption of NIR fluorescence creates a high signal-to-background ratio for intraoperative identification (60). The NIR fluorescence imaging systems can simultaneously acquire white light imaging and detect narrow spectrum ICG/NIR fluorescence (Figure 4) (61).
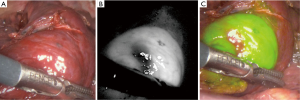
In a clinical trial of transthoracic administration of ICG conducted in the HOR at our institution, Ujiie et al. demonstrated a 90% (18/20) successful NIR-fluorescence detection rate of ICG-labeled pulmonary nodules (Table 2). One undetected lesion was located 48mm from the visceral pleura; the other was in a patient in whom intraoperative lung deflation was found to be incomplete (16).
The ICG/NIR fluorescence detection rate is limited by its capacity for tissue penetration, which would be affected by nodule depth from the visceral pleura or inflation status of the parenchyma (16).
99mTc-MAA
Radiotracers are unaffected by nodular depth, and thus have been a focus of investigation for localization of ill-defined pulmonary nodules. Work thus far has shown these agents to be safe and effective (18,62). Once the introducer needle is positioned under MDCT guidance, 0.1 mL Technetium-macro-aggregated albumin (99mTc-MAA) (MAASOL, GE Healthcare, Chicago, IL, USA) is injected into the lung parenchyma adjacent to target lesions (62,63). The patient is then transferred to the nuclear medicine department for scintigraphy to confirm successful injection of the radiolabeled agent. The radiotracer-labeled areas are located intraoperatively using an endoscopic gamma probe (Daniel Lung Probe, Dilon Diagnostics, Newport News, VA, USA) placed in contact with the lung surface (63). After resection, the gamma probe should be used again to ensure no residual radiotracer remains in the residual lung (62).
Though there is no available study regarding applying radio-guided pulmonary resection in HOR settings specifically, Polites et al. has demonstrated this approach in patients with previously diagnosed extra-thoracic malignancy (63). The authors reported a 96% (23/24) successful detection rate of 99mTc-MAA-labeled pulmonary nodules. One 99mTc-MAA-labeled nodule found undetectable by preoperative scintigraphy was subsequently identified by palpation after thoracotomy (63).
Limitations of radiotracer-guided localization include (I) possible intra-alveolar diffusion or pleural extravasation of radiotracer, (II) additional intervention time associated with the preoperative scintigraphy (64), (III) the accuracy could be affected by prolonged interval time regarding the 6.02 h half-life of 99mTc (18,19).
Combining marking techniques
Given that each fiducial material has inherent benefits and limitations, several authors have proposed combinations of marking techniques for thoracoscopic removal of ill-defined pulmonary nodules. In one study, pulmonary nodules were marked with both ICG and a micro-coil. For undetected ICG-labeled targets, thoracoscopic resection was based on subsequent localization with micro-coil under the intraoperative fluoroscopy guidance (16). Chao et al. proposed patient-tailored marking techniques according to the pleural depth of targets, e.g., hook-wire being used to mark target lesions located deeper than 20mm from the visceral pleura; PBV dye or ICG for the remainder (35). The authors demonstrated a 94% (94/100) success rate with this approach (Table 2). Localization failed in 6 patients: two had significant pneumothorax resulting from repeated percutaneous marking procedures; four experienced wire dislodgement or dye leakage (35).
Although the combination of marking techniques could potentially improve the rate of successful surgical resection, the approach remains limited by inherent complications from the transthoracic localization approach and fiducial material-specific disadvantages.
Other localization approaches suitable for pulmonary metastasectomy
Navigation bronchoscopy
Transbronchial placement of fiducial materials is potentially superior to transthoracic approach in several respects, including (I) avoidance of visceral pleura injury-related complications (65), (II) ability for marking of multiple lesions during one procedure (24,66), and (III) improved access to some regions of the lung that are potentially difficult or dangerous for a transthoracic approach (e.g., apical lung underneath the scapula, medial lung adjacent to vital organs, basal lung adjacent to the diaphragm) (67). The electromagnetic navigation system (SuperDimension; Medtronic, Minneapolis, MN, USA) allows the operator to utilize CT-reconstructed virtual endobronchial routes to target lesions via registration of the electromagnetic bronchoscopy to the electromagnetic tracking system (68,69). In HORs, the fluoroscopic view augmented with 3D imaging generated from intraoperative CBCT acquisition could further improve the accuracy of electromagnetic navigation; this is of particular importance to transbronchial biopsy and localization-guided resection (34,48,70).
Systemic administration of ICG—the enhanced permeability and retention effect
In a patient with extra-thoracic malignancy, there are often multiple pulmonary nodules that are concerning for metastasis (24). When there are multiple targets required labeling, it may be impractical or risky to perform repeated marking procedures, either by transthoracic or transbronchial approaches.
Angiogenesis factors released from malignant tumor cells can induce extensive neo-vascularization with defective endothelial structures, resulting in porous vascular walls. The enhanced permeability and retention (EPR) effect describes the non-specific passive leakage of nanoparticles from the bloodstream via fenestrated vasculature and subsequent accumulation in tumor tissue due to limited lymphatic drainage (71). When high doses of ICG (e.g., 3–5 mg/kg) is intravenously administrated, circulating ICG-lipoprotein particles gradually leak and accumulate into the tumor tissue via this EPR effect (17,72). The tumor-trophic accumulation of ICG allows NIR fluorescence imaging hours after infusion which allows intraoperative detection of malignancies (17,73).
Keating et al. reported an 81.8% (9/11) in vivo NIR fluorescence detection rate of pulmonary metastatic nodules by systemic ICG injection (5 mg/kg 24 hours before surgery). All detected nodules were located within 10 mm from the pleural surface; the undetected nodules were located at a depth of 16 and 18 mm, respectively (74). Based on results from previous clinical trials and this study, primary and secondary thoracic malignancies share the same EPR effect and thus the same ICG tumor-trophic accumulation (74,75).
Systemic injection of ICG could avoid inherent complications associated with transthoracic or transbronchial approaches, namely lung puncture injuries, fiducial material dislodgement, and repeated marking procedures if multiple pulmonary nodules are to be resected (76). The technique was unaffected by nodule size, metabolic activity, histology, or vascularity (75). Unfortunately, the capabilities of ICG/NIR fluorescence in vivo are ultimately limited by tissue penetration by NIR light (75) and non-specific tissue accumulation in certain conditions, such as inflammation (77). Molecular-targeted contrast agents, specific to the patient’s underlying tumor biology, represent an important step in the development of next-generation localization procedures (76,78).
Conclusions
Various localization approaches have been proposed to augment a surgeon’s ability to achieve complete surgical radicality during PME. Multimodality HORs permit on-site image acquisition and real-time intraoperative guidance for image-guided localization followed by thoracoscopic surgery. Selecting an appropriate fiducial marker based on the target nodule’s properties and available resources is critical for effective image-guided PME. The future of iVATS will likely focus on improving the tumor specificity of targeted contrast agents, allowing for improved accuracy for in vivo tumor identification and resection.
Acknowledgments
Z Chen received funding from Mackay memorial hospital (Taipei) and would like to thank the institution for their support.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Michel Gonzalez) for the series “Advancement in the Surgical Treatment of Pulmonary Metastasis” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2019.05.04). The series “Advancement in the Surgical Treatment of Pulmonary Metastasis” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bartlett EK, Simmons KD, Wachtel H, et al. The rise in metastasectomy across cancer types over the past decade. Cancer 2015;121:747-57. [Crossref] [PubMed]
- Treasure T, Milosevic M, Fiorentino F, et al. Pulmonary metastasectomy: what is the practice and where is the evidence for effectiveness? Thorax 2014;69:946-9. [Crossref] [PubMed]
- Erhunmwunsee L, Tong BC. Preoperative Evaluation and Indications for Pulmonary Metastasectomy. Thorac Surg Clin 2016;26:7-12. [Crossref] [PubMed]
- Thomford NR, Woolner LB, Clagett OT. The surgical treatment of metastatic tumors in the lungs. J Thorac Cardiovasc Surg 1965;49:357-63. [PubMed]
- Pastorino U, Buyse M, Friedel G, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg 1997;113:37-49. [Crossref] [PubMed]
- Suzuki K, Nagai K, Yoshida J, et al. Video-assisted thoracoscopic surgery for small indeterminate pulmonary nodules: indications for preoperative marking. Chest 1999;115:563-8. [Crossref] [PubMed]
- Tamura M, Oda M, Fujimori H, et al. New indication for preoperative marking of small peripheral pulmonary nodules in thoracoscopic surgery. Interact Cardiovasc Thorac Surg 2010;11:590-3. [Crossref] [PubMed]
- Nakashima S, Watanabe A, Obama T, et al. Need for preoperative computed tomography-guided localization in video-assisted thoracoscopic surgery pulmonary resections of metastatic pulmonary nodules. Ann Thorac Surg 2010;89:212-8. [Crossref] [PubMed]
- Yao F, Wang J, Yao J, et al. Reevaluation of the efficacy of preoperative computed tomography-guided hook wire localization: A retrospective analysis. Int J Surg 2018;51:24-30. [Crossref] [PubMed]
- Li C, Liu B, Jia H, et al. Computed tomography-guided hook wire localization facilitates vid-o-assisted thoracoscopic surgery of pulmonary ground-glass nodules. Thorac Cancer 2018;9:1145-50. [Crossref] [PubMed]
- Li F, Chen Y, Bian J, et al. Preoperative Computed Tomography-guided Microcoil Localization for Multiple Small Lung Nodules before Video-assisted Thoracoscopic Surgery. Zhongguo Fei Ai Za Zhi 2018;21:857-63. [PubMed]
- Lin MW, Tseng YH, Lee YF, et al. Computed tomography-guided patent blue vital dye localization of pulmonary nodules in uniportal thoracoscopy. J Thorac Cardiovasc Surg 2016;152:535-44.e2. [Crossref] [PubMed]
- Lee NK, Park CM, Kang CH, et al. CT-guided percutaneous transthoracic localization of pulmonary nodules prior to video-assisted thoracoscopic surgery using barium suspension. Korean J Radiol 2012;13:694-701. [Crossref] [PubMed]
- Watanabe K, Nomori H, Ohtsuka T, et al. Usefulness and complications of computed to-mography-guided lipiodol marking for fluoroscopy-assisted thoracoscopic resection of small pulmonary nodules: experience with 174 nodules. J Thorac Cardiovasc Surg 2006;132:320-4. [Crossref] [PubMed]
- Kawanaka K, Nomori H, Mori T, et al. Marking of small pulmonary nodules before thoracoscopic resection: injection of lipiodol under CT-fluoroscopic guidance. Acad Radiol 2009;16:39-45. [Crossref] [PubMed]
- Ujiie H, Kato T, Hu HP, et al. A novel minimally invasive near-infrared thoracoscopic localization technique of small pulmonary nodules: A phase I feasibility trial. J Thorac Cardiovasc Surg 2017;154:702-11. [Crossref] [PubMed]
- Mao Y, Chi C, Yang F, et al. The identification of sub-centimetre nodules by near-infrared fluorescence thoracoscopic systems in pulmonary resection surgeries. Eur J Cardiothorac Surg 2017;52:1190-6. [Crossref] [PubMed]
- Bellomi M, Veronesi G, Trifiro G, et al. Computed tomography-guided preoperative radio-tracer localization of nonpalpable lung nodules. Ann Thorac Surg 2010;90:1759-64. [Crossref] [PubMed]
- Galetta D, Bellomi M, Grana C, et al. Radio-Guided Localization and Resection of Small or Ill-Defined Pulmonary Lesions. Ann Thorac Surg 2015;100:1175-80. [Crossref] [PubMed]
- Manca G, Davini F, Tardelli E, et al. Clinical Impact of Radioguided Localization in the Treatment of Solitary Pulmonary Nodule: A 20-Year Retrospective Analysis. Clin Nucl Med 2018;43:317-22. [PubMed]
- Ujiie H, Effat A, Yasufuku K. Image-guided thoracic surgery in the hybrid operation room. J Vis Surg 2017;3:148. [Crossref] [PubMed]
- Gill RR, Zheng Y, Barlow JS, et al. Image-guided video assisted thoracoscopic surgery (iVATS) - phase I-II clinical trial. J Surg Oncol 2015;112:18-25. [Crossref] [PubMed]
- Davis SD. CT evaluation for pulmonary metastases in patients with extrathoracic malignancy. Radiology 1991;180:1-12. [Crossref] [PubMed]
- Caparica R, Mak MP, Rocha CH, et al. Pulmonary Nodules in Patients With Nonpulmonary Cancer: Not Always Metastases. J Glob Oncol 2016;2:138-144. [Crossref] [PubMed]
- Puchalski J. Pulmonary Manifestations of Solid Non-Pulmonary Malignancies. Clin Chest Med 2017;38:177-86. [Crossref] [PubMed]
- Whitesell PL, Peters SG. Pulmonary manifestations of extrathoracic malignant lesions. Mayo Clin Proc 1993;68:483-91. [Crossref] [PubMed]
- Seo JB, Im JG, Goo JM, et al. Atypical pulmonary metastases: spectrum of radiologic findings. Radiographics 2001;21:403-17. [Crossref] [PubMed]
- Wang Memoli JS, Nietert PJ, Silvestri GA. Meta-analysis of guided bronchoscopy for the evaluation of the pulmonary nodule. Chest 2012;142:385-93. [Crossref] [PubMed]
- DiBardino DM, Yarmus LB, Semaan RW. Transthoracic needle biopsy of the lung. J Thorac Dis 2015;7:S304-16. [PubMed]
- Kang HS, Ha JH, Kang HH, et al. Factors Related to the Diagnostic Yield of Flexible Bronchoscopy without Guidance in Bronchoscopically Invisible Peripheral Lung Lesions. Tuberc Respir Dis (Seoul) 2017;80:284-90. [Crossref] [PubMed]
- Mohammed TL, Chowdhry A, Reddy GP, et al. ACR Appropriateness Criteria(R) screening for pulmonary metastases. J Thorac Imaging 2011;26:W1-3. [Crossref] [PubMed]
- Petrella F, Diotti C, Rimessi A, et al. Pulmonary metastasectomy: an overview. J Thorac Dis 2017;9:S1291-8. [Crossref] [PubMed]
- Welter S, Arfanis E, Christoph D, et al. Growth patterns of pulmonary metastases: should we adjust resection techniques to primary histology and size? Eur J Cardiothorac Surg 2017;52:39-46. [PubMed]
- Ng CSH, Chu CM, Lo CK, et al. Hybrid operating room Dyna-computed tomography combined image-guided electromagnetic navigation bronchoscopy dye marking and hookwire localization video-assisted thoracic surgery metastasectomy. Interact Cardiovasc Thorac Surg 2018;26:338-40. [Crossref] [PubMed]
- Chao YK, Wen CT, Fang HY, et al. A single-center experience of 100 image-guided video-assisted thoracoscopic surgery procedures. J Thorac Dis 2018;10:S1624-30. [Crossref] [PubMed]
- Chao YK, Pan KT, Wen CT, et al. A comparison of efficacy and safety of preoperative versus intraoperative computed tomography-guided thoracoscopic lung resection. J Thorac Cardiovasc Surg 2018;156:1974-83.e1. [Crossref] [PubMed]
- van der Bruggen-Bogaarts BA, Broerse JJ, Lammers JW, et al. Radiation exposure in standard and high-resolution chest CT scans. Chest 1995;107:113-5. [Crossref] [PubMed]
- Chen PH, Hsu HH, Yang SM, et al. Preoperative Dye Localization for Thoracoscopic Lung Surgery: Hybrid Versus Computed Tomography Room. Ann Thorac Surg 2018;106:1661-7. [Crossref] [PubMed]
- Cameron RB. Interventional radiology suite or hybrid operating room: Which is the best for lung nodule localization? J Thorac Cardiovasc Surg 2018;156:1984-5. [Crossref] [PubMed]
- Hsieh MJ, Wen CT, Fang HY, et al. Learning curve of image-guided video-assisted thoracoscopic surgery for small pulmonary nodules: A prospective analysis of 30 initial patients. J Thorac Cardiovasc Surg 2018;155:1825-32.e1. [Crossref] [PubMed]
- Fumimoto S, Sato K, Koyama M, et al. Combined lipiodol marking and video-assisted thoracoscopic surgery in a hybrid operating room. J Thorac Dis 2018;10:2940-7. [Crossref] [PubMed]
- Ohtaka K, Takahashi Y, Kaga K, et al. Video-assisted thoracoscopic surgery using mobile computed tomography: new method for locating of small lung nodules. J Cardiothorac Surg 2014;9:110. [Crossref] [PubMed]
- Kawada M, Okubo T, Poudel S, et al. A new marking technique for peripheral lung nodules avoiding pleural puncture: the intrathoracic stamping method. Interact Cardiovasc Thorac Surg 2013;16:381-3. [Crossref] [PubMed]
- Siewerdsen JH, Daly MJ, Chan H, et al. High-performance intraoperative cone-beam CT on a mobile C-arm: an integrated system for guidance of head and neck surgery. In: Miga MI, Wong KH. editors. Medical Imaging 2009: Visualization, Image-Guided Procedures, and Modeling. Lake Buena Vista: SPIE, 2009;72610-8.
- Chen Z, Ujiie H, Gregor A, et al. CBCT-guided micro-coil placement. Asvide 2019;6:136. Available online: http://www.asvide.com/article/view/31898
- Yu PSY, Man Chu C, Lau RWH, et al. Video-assisted thoracic surgery for tiny pulmonary nodules with real-time image guidance in the hybrid theatre: the initial experience. J Thorac Dis 2018;10:2933-9. [Crossref] [PubMed]
- Yang SM, Ko WC, Lin MW, et al. Image-guided thoracoscopic surgery with dye localization in a hybrid operating room. J Thorac Dis 2016;8:S681-9. [Crossref] [PubMed]
- Pritchett MA, Schampaert S, de Groot JAH, et al. Cone-Beam CT With Augmented Fluoroscopy Combined With Electromagnetic Navigation Bronchoscopy for Biopsy of Pulmonary Nodules. J Bronchology Interv Pulmonol 2018;25:274-82. [Crossref] [PubMed]
- Hwang S, Kim TG, Song YG. Comparison of hook wire versus coil localization for video-assisted thoracoscopic surgery. Thorac Cancer 2018;9:384-9. [Crossref] [PubMed]
- Ichinose J, Kohno T, Fujimori S, et al. Efficacy and complications of computed tomography-guided hook wire localization. Ann Thorac Surg 2013;96:1203-8. [Crossref] [PubMed]
- Sakiyama S, Kondo K, Matsuoka H, et al. Fatal air embolism during computed tomography–guided pulmonary marking with a hook-type marker. J Thorac Cardiovasc Surg 2003;126:1207-9. [Crossref] [PubMed]
- Sancheti MS, Lee R, Ahmed SU, et al. Percutaneous fiducial localization for thoracoscopic wedge resection of small pulmonary nodules. Ann Thorac Surg 2014;97:1914-8; discussion 1919.
- Yi JH, Choi PJ, Bang JH, et al. Systemic air embolism after computed tomography-guided hook wire localization: two case reports and literature review. J Thorac Dis 2018;10:E59-64. [Crossref] [PubMed]
- McConnell PI, Feola GP, Meyers RL. Methylene blue-stained autologous blood for needle localization and thoracoscopic resection of deep pulmonary nodules. J Pediatr Surg 2002;37:1729-31. [Crossref] [PubMed]
- Chen JR, Tseng YH, Lin MW, et al. Safety and efficacy of computed tomography-guided dye localization using patent blue V for single lung nodule for video-assisted thoracoscopic surgery: a retrospective study. Ann Transl Med 2019;7:28. [Crossref] [PubMed]
- Mertes PM, Malinovsky JM, Mouton-Faivre C, et al. Anaphylaxis to dyes during the perioperative period: reports of 14 clinical cases. J Allergy Clin Immunol 2008;122:348-52. [Crossref] [PubMed]
- Wu TT, Chang YC, Lee JM, et al. Anaphylactic reaction to patent blue V used in preoperative computed tomography-guided dye localization of small lung nodules. J Formos Med Assoc 2016;115:288-9. [Crossref] [PubMed]
- Yamagami T, Yoshimatsu R, Miura H, et al. Pneumonia occurring after injection of Lipiodol to localize pulmonary nodules before fluoroscopy-aided thoracoscopic resection. Acta Radiol Short Rep 2014;3:2047981613499754 [Crossref] [PubMed]
- Schaafsma BE, Mieog JS, Hutteman M, et al. The clinical use of indocyanine green as a near-infrared fluorescent contrast agent for image-guided oncologic surgery. J Surg Oncol 2011;104:323-32. [Crossref] [PubMed]
- Matsui A, Tanaka E, Choi HS, et al. Real-time intra-operative near-infrared fluorescence identification of the extrahepatic bile ducts using clinically available contrast agents. Surgery 2010;148:87-95. [Crossref] [PubMed]
- Fengler J. Near-infrared fluorescence laparoscopy--technical description of PINPOINT(R) a novel and commercially available system. Colorectal Dis 2015;17:3-6. [Crossref] [PubMed]
- Stiles BM, Altes TA, Jones DR, et al. Clinical experience with radiotracer-guided thoracoscopic biopsy of small, indeterminate lung nodules. Ann Thorac Surg 2006;82:1191-6; discussion 6-7. [Crossref] [PubMed]
- Polites SF, Fahy AS, Sunnock WA, et al. Use of radiotracer labeling of pulmonary nodules to facilitate excisional biopsy and metastasectomy in children with solid tumors. J Pediatr Surg 2018;53:1369-73. [Crossref] [PubMed]
- Bertolaccini L, Salgarello M, Gorgoni G, et al. Radioguided video-assisted resection of non-palpable solitary pulmonary nodule/ground glass opacity: how to do it. J Vis Surg 2015;1:9. [PubMed]
- Anayama T, Qiu J, Chan H, et al. Localization of pulmonary nodules using navigation bronchoscope and a near-infrared fluorescence thoracoscope. Ann Thorac Surg 2015;99:224-30. [Crossref] [PubMed]
- Anayama T, Hirohashi K, Miyazaki R, et al. Near-infrared dye marking for thoracoscopic re-section of small-sized pulmonary nodules: comparison of percutaneous and bronchoscopic injection techniques. J Cardiothorac Surg 2018;13:5. [Crossref] [PubMed]
- Lin MW, Chen JS. Image-guided techniques for localizing pulmonary nodules in thoracoscopic surgery. J Thorac Dis 2016;8:S749-55. [Crossref] [PubMed]
- Abbas A, Kadakia S, Ambur V, et al. Intraoperative electromagnetic navigational bronchoscopic localization of small, deep, or subsolid pulmonary nodules. J Thorac Cardiovasc Surg 2017;153:1581-90. [Crossref] [PubMed]
- Marino KA, Sullivan JL, Weksler B. Electromagnetic Navigation Bronchoscopy for Identifying Lung Nodules for Thoracoscopic Resection. Ann Thorac Surg 2016;102:454-7. [Crossref] [PubMed]
- Anayama T, Hirohashi K, Okada H, et al. Simultaneous cone beam computed tomography-guided bronchoscopic marking and video-assisted thoracoscopic wedge resection in a hybrid operating room. Thorac Cancer 2019;10:579-82. [Crossref] [PubMed]
- Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res 1986;46:6387-92. [PubMed]
- Newton AD, Predina JD, Corbett CJ, et al. Optimization of Second Window Indocyanine Green for Intraoperative Near-Infrared Imaging of Thoracic Malignancy. J Am Coll Surg 2019;228:188-97. [Crossref] [PubMed]
- Madajewski B, Judy BF, Mouchli A, et al. Intraoperative near-infrared imaging of surgical wounds after tumor resections can detect residual disease. Clin Cancer Res 2012;18:5741-51. [Crossref] [PubMed]
- Keating J, Newton A, Venegas O, et al. Near-Infrared Intraoperative Molecular Imaging Can Locate Metastases to the Lung. Ann Thorac Surg 2017;103:390-8. [Crossref] [PubMed]
- Okusanya OT, Holt D, Heitjan D, et al. Intraoperative near-infrared imaging can identify pulmonary nodules. Ann Thorac Surg 2014;98:1223-30. [Crossref] [PubMed]
- Nagaya T, Nakamura YA, Choyke PL, et al. Fluorescence-Guided Surgery. Front Oncol 2017;7:314. [Crossref] [PubMed]
- Kim HK, Quan YH, Choi BH, et al. Intraoperative pulmonary neoplasm identification using near-infrared fluorescence imaging. Eur J Cardiothorac Surg 2016;49:1497-502. [Crossref] [PubMed]
- Okusanya OT, DeJesus EM, Jiang JX, et al. Intraoperative molecular imaging can identify lung adenocarcinomas during pulmonary resection. J Thorac Cardiovasc Surg 2015;150:28-35.e1. [Crossref] [PubMed]
Cite this article as: Chen Z, Ujiie H, Gregor A, Bernards N, Yasufuku K. Image-guided pulmonary metastasectomy in the hybrid operating room. J Vis Surg 2019;5:51.


