
Minimalist three-dimensional thoracoscopic extended thymomectomy in a patient with myasthenia gravis
Introduction
Thymoma is the most common primary mediastinal neoplasm in adults. The majority of thymomas are benign and slow-growing although more invasive variants can more rarely be encountered (1). In about 35% of instances thymomas can be associated with myasthenia gravis (MG), an autoimmune disease that is characterized by muscle weakness and fatigue and in which thymectomy and/or thymomectomy can play a therapeutic role in association with pharmacological treatment (2).Therefore, in patients with thymoma and MG, radical thymomectomy together with complete removal of the thymus gland and of the perithymic fatty tissue has been considered essential both for oncological and symptomatic treatment (3).
Thymomectomy has been performed by video-assisted thoracic surgery (VATS) approaches with promising results (4). In addition, to further minimize the overall surgical trauma, thymomectomy has been recently performed through uniportal VATS and also by nonintubated anesthesia protocols (5).
In this setting, one limitation of standard VATS is the 2-dimensional (2D) flat view of the surgical field with lack of depth perception, potentially leading to image distortion, impaired hand-eye coordination, less meticulous dissection and decreased ability to reach the target (6). To overcome these limitations, three-dimensional (3D) image systems have been recently applied to VATS (7).
Herein, we report on a MG patient with invasive thymoma infiltrating the left lung who underwent a minimalist extended thymomectomy entailing a uniportal, nonintubated VATS resection of the tumor, infiltrated lung and perithymic fatty tissue using a 3D camera system.
Case presentation
Patient selection and workup
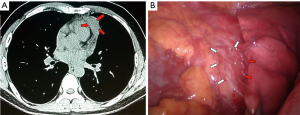
A 42 years old man with electromyography-based diagnosis of generalized myasthenia gravis since 2 months (class IIIA according to the classification of Myasthenia Gravis Foundation of America, positive anti-acetylcholine-receptor antibodies and evidence at computed tomography (CT) of a 4-cm in maximal size thymic mass, was scheduled for thoracoscopic thymomectomy (Figure 1). However, since myasthenic symptoms proved unstable and poorly controlled by pyridostigmine and corticosteroids, the patient underwent two cycles of preoperative plasmapheresis. After two weeks, a minimalist VATS extended thymomectomy entailing nonintubated anesthesia and a left-sided uniportal access was carried out.
Anesthesia
The nonintubated anesthesia protocol included thoracic analgesia assured by a simple intercostal block carried out by local anesthesia (40 mL of a 50% mixture of ropivacaine and bupivacaine) injected in a stepwise fashion starting with the subcutaneous tissue and proceeding with the intercostal muscles and beneath the parietal pleura. The patient was sedated by target control infusion of propofol optimized with bispectral index monitoring to keep adequate deepness of sedation while maintaining spontaneous ventilation. A laryngeal mask was placed to assist ventilation whenever necessary (5).
Equipment preference card
Here is a list of all equipment used during the procedure:
- 3D HD system with a 30-degree 10 mm camera (Vicking, Conmed, Utica, USA).
- Polarized lenses.
- Alexis soft tissue wound retractor (Alexis; Apllied Medical, Rancho Santa Margarita, Calif).
- Endoscopic cotton swabs.
- Endoscopic clip applier, 60 mm Echelon® endostapler, and Ultracision® Harmonic scalpel (all from Ethicon Endosurgery, Pomezia, Italy).
Surgical procedure
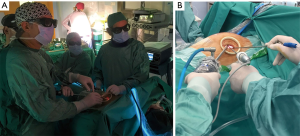
The patient was placed in a 30-degree semilateral position and through a single 3-cm incision in the fourth left intercostal space; a soft tissue retractor was inserted and 3D optic with a 30-degree angled camera was introduced (Figure 2). The thymic mass appeared to be invading the left upper lobe of the lung leading us to start the surgical procedure with a wedge resection of the lung en bloc with the mass by a 60 mm endostapler (Figure 3). Subsequently, complete resection of the mass including the thymus gland and perithymic fatty tissue comprised within the anterior-superior mediastinum was carried out between both phrenic nerves laterally starting from the lower surface of the thymoma and proceeding towards the right side using for dissection maneuvres either endoscopic cotton swabs and harmonic scalpel. Following complete isolation of the tumor, we decided to resect it firstly en bloc with the lung because of its large size in order to facilitate the subsequent completion of thymectomy and to avoid excessive tumor manipulation. Thymectomy was thus completed by progressive isolation of both lower and upper thymic horns and interruption by endoscopic metal clips of the thymic veins. Afterwards, all the extra-thymic fatty tissue including the pericardial and cardiophrenic fat was totally removed in order to accomplish a formal extended thymectomy. Finally a 28-F chest drain was inserted and the lung was re-inflated. Closure of the muscle, subcutaneous and skin was carried out in layers.

Role of team members
The team consisted of two thoracic surgeons, one thoracic surgery resident, two anesthesiologists, and one medical student trainee. One surgeon performed all the steps of the procedure with the assistance of the other surgeon and the resident was the camera assistant during the entire procedure.
Postoperative management
The overall operative time was 100 minutes without any intraoperative complications. Few minutes later the patient was fully awake and comfortably able to talk clearly and move his arms with no pain (Figure 3). There were no post-operative complications and on day one postoperatively, the chest drain was removed and the patient was discharged.
Histopathological examination showed a WHO B2 thymoma infiltrating the lung and extracapsular fatty tissue with free resection margins. Twelve months after surgery the patient has improved in MG symptoms and is free of recurrence on PET-CT scan.
Tips, tricks and pitfalls
Planning of uniportal thymomectomy with use of 3D camera system in nonintubated patients requires attention to few main details:
- Sometimes neither preoperative CT nor magnetic resonance imaging allow to recognize a tumor infiltration of surrounding structures. This feature emphasizes the importance of an initial careful intraoperative exploration of peri-tumoral anatomical structures.
- During VATS thymomectomy/thymectomy complete instrumentation for emergency conversion to median sternotomy including sternal retractor and electric saw should be ready on the table and it is better to set the patient positioning in a way that total median sternotomy can be done by just rotating the table in order to place the patient supine.
- Nonintubated anaesthesia protocol for thymectomy/thymomectomy should include insertion of a laryngeal mask to allow assistance of ventilation in case of inadvertent or deliberate opening of both mediastinal pleurae, a condition which might jeopardize spontaneous ventilation due to a loss of an inspiratory pressure gradient.
- In patients with MG we now prefer to avoid use of muscle relaxants to minimize risks of symptoms deterioration in the immediate postoperative period.
- By using the 3D system, care must be taken to avoid excessive movements of the camera, which can cause discomfort to the surgeons during surgical manoeuvring.
- When using the 3D camera system we have noticed that the lens must be cleaned more often than with 2D systems.
- We adopt a uniportal VATS approach for thymomas with maximal size ≤5 cm whereas for larger thymomas we more commonly employ median sternotomy. Moreover, in presence of particularly large and space occupying lesions, we prefer to isolate and remove first the tumor to facilitate subsequent isolation of the rest of the thymus gland.
- For sharp maneuvers and coagulation we routinely employ the harmonic scalpel instead of standard electrocautery since it produces less smoke and eliminates risk of triggering cardiac arrhythmias.
Discussion
The association of the thymus with MG has been suggested by the evidence that about one-third of patients with thymoma develop MG, while about 10% of MG patients have a thymoma (2).
Complete resection of thymoma indicating removal of the tumor along with any invaded adjacent tissues, has shown to be an important prognostic factor associated with better survival and lower recurrence rates (3). There are different surgical approaches for thymomectomy including open transcervical, trans-sternal and combined approaches, as well as several VATS and robotic alternatives (4). In a recent report by Burt et al. (9), VATS was the most common minimally invasive technique employed for thymomectomy (68%).
Over the last decades, both VATS and robotic approaches have been increasingly applied for thymomectomy (10). Compared to open surgery, VATS thymomectomy has been showing similar results in terms of overall recurrence and free survival rates with lower complications and shorter hospital stays (9,11) although more data with long-term follow-up are needed. The conventional 2D visual systems lack depth perception, which can affect accurate judgment of the distance between the endoscopic instruments and the target tissue causing also difficulty in hand-eye coordination (6). To overcome these disadvantages, 3D endoscopic systems have been adopted both for VATS and robotic approaches. Potential advantages of VATS over robot-assisted surgery include lower costs, faster learning curve and superior possibility of tissue palpation whereas potential disadvantages include inferior range of mobility of instrumentation and less precise fine surgical manoeuvring (10).
Three-D systems have attracted thoracic surgeons as it magnifies the field and provides a better sense of depth, which can facilitate precise surgical manoeuvring (12). On the other hand, reported limitations of 3D endoscopic systems include the difficulty to rotate the lens separately, the heavy weight of the hand shank which may be a burden for the assistant. Also, the 3D systems can magnify the image by ten times, while the 2D systems-related magnification is of about four times. As a result with the 3D systems, the visible surgical field is more limited though more magnified, camera movements have to be slow to avoid to lose a clear vision of the field, and the optic needs to be cleaned more often (12,13). However, we have found that gaining confidence with 3D VATS can mitigate most of adverse effects except for the need to clean the lens quite frequently.
Jiang et al. (14) reported about nonintubated subxiphoid 3D-VATS resection of a 3cm thymoma using a novel glasses-free display system that allows surgeons to operate with high image quality without polarized lenses. In fact, the adoption of a subxiphoid approach provides an excellent view of both pleural cavities, which can aid the complete removal of pericardial and diaphragmatic fatty tissue although we believe that it can render more difficult to dissect free the superior thymic horns up in the lower cervical region.
In our uniportal VATS thymomectomy that we denominated minimalist, we preferred use of local anesthesia under spontaneous ventilation with avoidance of muscle relaxants. This in an attempt of reducing the adverse effects of tracheal intubation and drug-induced muscle paralysis. It is worth noting that the surgical view resulting from our nonintubated anaesthesia protocol proved similar to that achieved by single-lung ventilation. In fact collapse of the nondependent lung was nearly complete whereas adoption of a laryngeal mask added the possibility to assist ventilation in case of opening of both pleural cavities, and to reduce the degrees of permissive hypercapnia, which is related to the pendular ventilation that characterizes all VATS procedures performed under spontaneous ventilation. We also believe that adoption this type of anaesthesia might facilitate an early recovery in delicate patients such as patients with MG in whom it might decrease the risk of myasthenic crisis (4,5).
Nonetheless, potential shortcomings of a nonintubated anesthesia protocol include the possibility that diaphragmatic and/or lung movements might disturb somewhat surgical maneuvring and that incidental occurrence of major vascular injury may result in some delay in prompt management due to the need of conversion to tracheal intubation and emergency median sternotomy, which highlight the need of adequate training of the surgical/anesthesiological teams involved in this type of surgery.
In conclusion, a minimalist VATS extended thymomectomy carried out by a 3D display system proved safe and easily feasible in our patient. We believe that the adoption of the 3D display systems can provide better depth of perception and more magnified surgical view, which might help surgeons to work more comfortably and more precisely, particularly within anterior superior mediastinum structures.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2019.04.05). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in studies involving human participants were in accordance with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Davenport E, Malthaner RA. The role of surgery in the management of thymoma: a systematic review. Ann Thorac Surg 2008;86:673-84. [Crossref] [PubMed]
- Kondo K, Monden Y. Myasthenia gravis appearing after thymectomy for thymoma. Eur J Cardiothorac Surg 2005;28:22-5. [Crossref] [PubMed]
- Detterbeck F, Moran C, Huang J, et al. Which way is up? Policies and procedures for surgeons and pathologist regarding resection specimens of thymic malignancy. J Thorac Oncol 2011;6:1730-8. [Crossref]
- Pompeo E, Elkhouly AG. Spontaneous ventilation thoracoscopic thymectomy: attractive or exceptionable?. J Thorac Dis 2018;10:S3981-3. [Crossref] [PubMed]
- Pompeo E, Dauri M, Massa R, et al. Minimalist thoracoscopic resection of thymoma associated with myasthenia gravis. J Thorac Cardiovasc Surg 2017;154:1463-5. [Crossref] [PubMed]
- Divisi D, Barone M, Crisci R. Three-dimensional video-assisted thoracic surgery for pulmonary resections: an update. J Vis Surg 2017;3:79. [Crossref] [PubMed]
- Gonzalez-Rivas D, Yang Y, Ng C. Advances in uniportal video-assisted thoracoscopic surgery: pushing the envelope. Thorac Surg Clin 2016;26:187-201. [Crossref] [PubMed]
- Elkhouly AG, Cristino B, Alhasan A, et al. The videoclip shows in a 2D format the actual 3D surgical procedure. Asvide 2019;6:131. Available online: http://www.asvide.com/article/view/31641
- Burt BM, Yao X, Shrager J, et al. Determinants of complete resection of thymoma by minimally invasive and open thymectomy: analysis of an international registry. J Thorac Oncol 2017;12:129-36. [Crossref] [PubMed]
- Mussi A, Fanucchi O, Davini F, et al. Robotic extended thymectomy for early-stage thymomas. Eur J Cardiothorac Surg 2012;41:e43-6. [Crossref] [PubMed]
- Friedant AJ, Handorf EA, Su S, et al. Minimally invasive versus open thymectomy for thymic malignancies: systematic review and meta-analysis. J Thorac Oncol 2016;11:30-8. [Crossref] [PubMed]
- Jiao P, Wu Q, Sun Y, et al. Comparative study of three-dimensional versus two-dimensional video-assisted thoracoscopic two-port lobectomy. Thorac Cancer 2017;8:3-7. [Crossref] [PubMed]
- Liu J, Cui F, Li J, et al. Development and clinical applications of glasses-free three-dimensional (3D) display technology for thoracoscopic surgery. Ann Transl Med 2018;6:214. [Crossref] [PubMed]
- Jiang L, Liu J, Shao W, et al. Non-intubated subxiphoid uniportal video-assisted thoracoscopic thymectomy using glasses-free 3D vision. J Thorac Dis 2016;8:E1602-4. [Crossref] [PubMed]
Cite this article as: Elkhouly AG, Cristino B, Alhasan A, Dauri M, Pompeo E. Minimalist three-dimensional thoracoscopic extended thymomectomy in a patient with myasthenia gravis. J Vis Surg 2019;5:49.


