Emerging biomarkers in pulmonary metastasectomy
Introduction
Current management of patients with pulmonary metastases comprises various therapeutic approaches including chemotherapy, targeted therapy, external radiotherapy, radiofrequency ablation and pulmonary metastasectomy (PM) (1). Beside recent advances among all of these options, PM still seems to provide the best achievable long-term outcome for patients with lung metastasis (2-4). Currently established selection criteria for PM include (I) control of the primary tumor, (II) absence of extrathoracic metastasis, (III) resectability of all metastatic lesions with adequate postoperative pulmonary reserve and (IV) absence of alternative medical treatment options with lower morbidity (5). In carefully selected patients, 5-year survival rates up to 70% have been reported and clinical outcome after PM has been constantly improving over the last decades (6). However, there is a great variation in long-term prognosis with regard to recurrence and survival rates after PM (7,8). This variation might also be due to the limitations of currently used selection criteria, which are mainly based on clinical factors without taking the biology of the primary tumor and the metastatic spread into account. Therefore, identifying patients who will benefit most from a surgical procedure in malignant disease with systemic spread remains a major obstacle.
Particularly in times of personalized medicine, knowledge on the tumor biology is essential for offering effective and evidence-based therapies. In many neoplastic diseases, prognostic and predictive biomarkers are becoming an integral part of guidelines and play an essential role in selecting candidates for individualized treatment algorithms (9). In context with PM, numerous biomarkers with remarkable prognostic and predictive potential have been reported over the last decades. However, the majority of these biomarkers have been identified in small series, from different centers and from different primary entities. Surveys amongst physicians on the current practice showed that the clinical application of these biomarkers in the setting of PM is still limited (10,11).
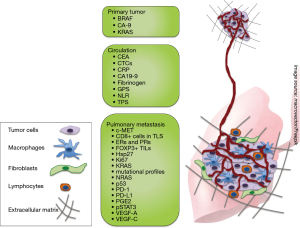
In 2013, our group summarized the evidence for molecular markers in patients with pulmonary metastasis (12). This review is an update to our previous review and summarizes recently available literature on biomarkers in the context of PM since 2013. It provides comprehensive up-to-date summary for medical professionals treating patients with lung metastasis as well as for researchers in the field of translational oncology. Biomarkers were assigned to three different groups, depending on the origin of the sample: (I) primary tumor; (II) circulating markers and (III) pulmonary metastasis (Figure 1).
Biomarkers detected in corresponding primary tumors
Primary tumor biomarkers can be detected within resected tissue specimen of different primary entities (Table 1). They may be especially useful when selecting between good and poor candidates for PM. In general, the concordance between histological markers in the primary tumors compared to pulmonary metastases is poor. This might be attributed to the evolution of tumor cells during the metastatic process (17).
Table 1
| Biomarker | Cut-off | Impact | Sample size | Primary entity | Reference |
|---|---|---|---|---|---|
| BRAF | V600 mutation |
OS ↓ | 180 | CRC | Renaud |
| CA-9 | High |
Recurrence ↓ | 44 | CRC | Schweiger |
| KRAS | KRAS mutation |
Recurrence ↓ | 494 | CRC | Pereira |
| KRAS | KRAS mutation |
OS ↓ | 75 | CRC | Ghidini |
↓, decreased. BRAF, v-raf murine sarcoma viral oncogene homolog B1; CA-9, Carbonic anhydrase IX; KRAS, Kirsten rat sarcoma viral oncogene homologue; OS, overall survival.
BRAF
The v-raf murine sarcoma viral oncogene homolog B1 (BRAF) is a well-known human proto-oncogene on chromosome 7, encoding for the serin/threonine kinase called B-Raf. B-Raf plays a key-role in the mitogen-activated protein kinase (MAPK) signaling pathway, regulating normal cellular growth and cell-division (18). Mutations of the B-raf cascade are commonly found in different human cancer types and already target of novel inhibitory drugs (19,20). Renaud et al. demonstrated the prognostic value of BRAF in patients with PM from metastatic colorectal cancer (mCRC) (13). In a cohort of 180 patients and subsequent PM, 19 patients had mutated BRAF (V600) in primary CRC specimen and showed significantly worse OS when compared to those patients with wild-type BRAF (5-year survival 0% vs. 100%).
Carbonic anhydrase IX (CA-9)
Hypoxia increases the expression of CA-9, an enzyme that catalyzes key-steps in metabolic processes and particularly in pH regulation. Overexpression of CA-9 was reported to be associated with poor outcome in different malignancies (21-24). It was demonstrated that patients with a high expression of CA-9 in resected primary CRC specimen had significantly earlier (23 vs. 37 months) spreading to the lung (14).
KRAS
As a part of the Ras family, the proto-oncogene KRAS encodes for a protein called K-ras that functions as an essential component in cell proliferation signaling. Mutations of KRAS can lead to constitutive overactivation of proliferation pathways, promoting tumorigenesis and metastasis (25-27). In a retrospectively analyzed cohort of 494 patients with mCRC and lung metastasis, 202 patients (41%) had KRAS mutations within the primary tumor tissue (15). Pereira et al. found that in case of a KRAS mutation the time to pulmonary metastasis was significantly decreased (15.2 vs. 22.4 months). Ghidini et al. further supplemented these findings by showing that patients with KRAS mutation in primary CRC specimen have significantly shorter (36.6 vs. 60.9 months) overall survival (OS) when undergoing PM (16). Renaud et al. supported these findings by demonstrating poor OS (5-year OS 44% vs. 100%) after PM in case of KRAS mutation in corresponding CRC specimen (13).
Circulating biomarkers
Circulating biomarkers are of increasing interest as they can be easily obtained from peripheral blood samples (Table 2). Circulating tumor markers e.g., CEA and CA 19-9 are already well established and have a number of clinical applications. In patients with lung metastasis, they might play a role for predictive purposes when selecting patients for surgery as well as for analysis of recurrence patterns after PM.
Table 2
| Biomarker | Cut-off | Impact | Sample size | Primary entity | Reference |
|---|---|---|---|---|---|
| CEA | >6 ng/mL | DFS ↓ | 84 | CRC | Cho |
| CEA | >5 ng/mL | OS ↓ | 522 | CRC | Embun |
| CEA | >5 ng/mL | OS ↓ | 154 | CRC | Sun |
| CEA | >5 ng/mL | OS ↓ | 33 | CRC | Hachimaru |
| CEA | >5 ng/mL | OS ↓ | 93 | CRC | Suzuki |
| CEA | >5 ng/mL | OS ↓ | 2,925 | CRC | Gonzalez |
| CEA | >5 ng/mL | DFS, OS ↓ | 59 | CRC | Yokoyama |
| CEA | >5 ng/mL | DFS, OS ↓ | 785 | CRC | Okumura |
| CEA | >5 ng/mL (pre- and postoperative) | OS ↓ | 87 | CRC | Osoegawa |
| CTCs (peripheral) | ≥2 cells/7.5 mL blood | DFS, OS ↓ | 79 | CRC | Hashimoto |
| CTCs (pulmonary vein) | Presence |
Thoracic lymph node involvement | 24 | CRC | Le |
| CRP | ≥0.5 mg/dl | DFS ↓ | 52 | CRC | Ghanim |
| CRP | >10 mg/L | OS ↓ | 88 | CRC | Li |
| CRP | ≥0.5 mg/dL | OS ↓ | 23 | Urinary bladder/upper urinary tract cancer | Nakawaga |
| CRP | >2 (preoperative) and > 84 mg/L (postoperative) | OS ↓ | 846 | Different epithelial tumors, sarcomas and melanomas | Pastorino |
| CA19-9 | >28 IU/mL | OS ↓ | 75 | CRC | Vodicka |
| Fibrinogen | >325 mg/dL | Recurrence, OS ↓ | 51 | CRC | Ghanim |
| GPS | 1/2 |
OS ↓ | 51 | CRC | Ghanim |
| GPS | 1/2 |
OS ↓ | 132 | CRC | Kobayashi |
| NLR | >4,05 | Recurrence, OS ↓ | 574 | CRC | Renaud |
| NLR | >4 | Recurrence, OS ↓ | 47 | CRC | Ghanim |
| TPS | >140 IU/L | Recurrence, OS ↓ | 75 | CRC | Vodicka |
↓, decreased. CEA, carcinoembryonic antigen; CTCs, circulating tumor cells; CRP, C-reactive protein; CA19-9, carbohydrate antigen 19-9; GPS, Glasgow Prognostic Score; NLR, neutrophil-to-lymphocyte ratio; TPS, tissue polypeptide specific antigen; DFS, disease-free-survival; OS, overall survival.
CEA
The carcinoembryonic antigen (CEA) is a glycoprotein involved in cell adhesion during fetal development and therefore hardly measurable in blood levels of healthy adults. However, increased serum levels of CEA have been reported in several cancer types and were associated with poor prognosis (46-48). In case of CRC pulmonary metastasis, a number of reports with small sample sizes showed that increased (>5 and >6 ng/mL) pre-thoracotomy levels of CEA correlate with worsened OS and early recurrence after PM (28,31,32,34,35). These findings were confirmed in a large systematic review and meta-analysis including 2,925 patients by Gonzalez et al., who found that an elevated (>5 ng/mL) pre-thoracotomy level of CEA represents a two-times higher risk of death (33). Furthermore, Sun et al. and Embun et al. pointed out that the prognostic value of CEA is even stronger when combined together with other discussed clinicopathological factors of poor survival (e.g., thoracic lymph node involvement, number of lung metastases, shorter disease-free survival after PM) (29,30). Interestingly, Osoegawa et al. highlighted that patients with elevated preoperative (>5 ng/mL) but normalized postoperative CEA levels have a better prognosis, compared to those patients whose CEA level remains high or even increase after PM (median survival time of 41.8 months compared with 28.1 or 15.7 months, respectively) (36).
Circulating tumor cells (CTCs)
CTCs are detectable in the blood stream of patients with malignant disease, especially in metastatic disease (49). They are extraordinary rare (on average one CTC per billion normal blood cells) and therefore hard to isolate, but seem to be a high-potential independent clinical prognostic marker in different cancers (50). Recently, two studies evaluating the role of CTCs in context with PM have been published. Hashimoto el al. showed, that 13 out of 79 enrolled mCRC patients had CTCs within preoperatively drawn peripheral blood samples (37). Patients with multiple CTCs (CTC count ≥2/7.5 mL blood) had a significant shorter disease-free survival (8.6 vs. 19.8 months) and OS (median OS not reached vs. 37.8 months). Le et al. analyzed blood samples drawn intraoperatively from the pulmonary vein in 24 patients undergoing PM with curative intent (38). They could isolate more CTCs in pulmonary venous blood samples than in peripherally taken blood samples at the same time. However, pulmonary venous CTC count did not correlate with clinical outcome results, but with the presence of positive thoracic lymph nodes.
C-reactive protein (CRP)
CRP is an established and widely used acute phase protein in current clinical routine. Elevated preoperative CRP serum levels were reported to predict poor outcome in different thoracic malignancies (51,52). In small sample sized cohorts including mCRC and urothelial carcinoma patients undergoing PM with curative intent, those patients with high preoperative CRP levels (≥0.5 or ≥1 mg/dL) suffered from significantly earlier tumor recurrence and worse OS (39-41). These findings were complemented in recent work including 846 consecutive patients with resectable lung metastasis from different primary malignancies. Pastorino et al. found out that elevated preoperative levels and postoperative levels of CRP together predict poor survival after PM, compared to low pre- and postoperative CRP levels (5-year OS 43% vs. 57%) (42).
Fibrinogen
Similarly to CRP, fibrinogen is another acute phase protein that had been recently investigated for its prognostic impact in context with PM. 78.4% of the assessed mCRC patients had elevated serum levels (cut-off 325 mg/dL) of fibrinogen one day before PM (39). High levels of fibrinogen predicted early tumor recurrence and were significantly associated with decreased OS (HR 10.745, 95% CI: 1.737–66.475).
Glasgow Prognostic Score (GPS)
The GPS is a systemic inflammation-based score (composed of serum elevation of CRP and decrease in albumin concentration) that has been reported to be significant as a prognostic indicator in various types of malignancies (53). Two recently published studies investigated the impact of GPS in context with PM (39,44). Kobayashi et al. reported that patients with elevated GPS scores had a significantly worse 5-year post-metastasectomy survival (13% vs. 46%) (44). Ghanim et al. confirmed this observation and found elevated GPS score to be an independent indicator of poor OS (39).
Neutrophil-to-lymphocyte ratio (NLR)
There is increasing evidence that neutrophils play a determining role in cancer progression and metastasis (54). Furthermore, they are able to suppress the anti-tumor effects of human lymphocytes (55). Consequently, an elevated serum NLR has been shown to associate with an adverse OS in numerous malignant diseases (56). In a recently published work by Renaud et al., 5-year survival of the 574 included patients undergoing PM with high NLR prior to surgery was 25%, compared to 83% in case of a low preoperative NLR (45). Furthermore, a high NLR was associated with a significantly decreased 5-year pulmonary-recurrence free survival (6% vs. 49%). Supporting these findings, Ghanim et al. presented poor OS and early recurrence in case of elevated preoperative NLR in a small cohort of 47 mCRC patients (39).
Carbohydrate antigen 19-9 (CA 19-9)
CA 19-9 is a glycoprotein currently used as a tumor-associated prognostic marker in the management of different gastrointestinal malignancies, especially of pancreatic cancer (57). In context with PM, Vodicka et al. found that CRC patients with pre-operatively elevated serum levels of CA 19-9 (cut-off >28 IU/mL) had a significantly higher risk of death (95% hazard ratio: 3.2, 95% confidence interval: 1.2–8.4) after surgical resection of pulmonary nodules (43).
Tissue polypeptide specific antigen
In contrast to other established biomarkers correlating with tumor burden, the tissue polypeptide specific antigen (TPS) particularly reflects the activity and proliferation-rate of cancer cells. Interestingly, a recently published work by Vodicka et al. delivered first results on the prognostic value of TPS in patients undergoing PM (43). High preoperative values of TPS (cut-off 140 IU/L) were associated with poor OS and early recurrence after PM.
Biomarkers detected in pulmonary metastasis
Biomarkers that are available within pulmonary metastasis specimen can be identified either after PM with curative intent or by a biopsy with diagnostic intent. Interestingly, they represent the largest group of biomarkers analyzed in context with PM within the last years (Table 3). In context to other non-surgical treatment modalities (e.g., radiofrequency-ablation or radiation) for pulmonary metastasis, it underlines the important role of surgery in providing adequate histological material for precise analysis and individualized patient management. Beyond the removal of all pulmonary nodules by means of a metastasectomy with curative intent, diagnostic metastasectomies are frequently performed to aid further treatment planning.
Table 3
| Biomarker | Cut-off | Impact | Sample size | Primary entity | Authors |
|---|---|---|---|---|---|
| c-MET | IHC Score >100 | OS ↓ | 51 | CRC | Schweiger |
| CD8+ cells in TLS | Low-expression | OS ↓ | 57 | CRC | Schweiger |
| ERs and PRs | Positive expression | OS ↑ | 81 | Breast cancer | Meimarakis |
| FOXP3+ TILs | Over-expression | OS ↑ | 57 | CRC | Schweiger |
| Hsp27 in stroma | Over-expression | Recurrence, OS ↓ | 51 | CRC | Schweiger |
| Ki67 | Staining area of ≥0.33 | OS ↓ | 29 | Osteosarcoma | Matsumoto |
| KRAS | Mutation |
Recurrence, OS ↓ | 44 | CRC | Schweiger |
| KRAS | exon 2 codon 12 |
Recurrence, OS ↓ | 150 | CRC | Renaud |
| EGFR, GNAQ, KIT, MET and PTPN11* | Mutation |
Recurrence ↓ | 47 | CRC | Schweiger |
| PDGFRA, SMARCB1, and TP53* | Mutation |
OS ↓ | 47 | CRC | Schweiger |
| NRAS | Mutation |
Recurrence, OS ↓ | 25 | Melanoma | Ulivieri |
| p53 | Over-expression | OS ↓ | 88 | CRC | Li |
| PD-1 | Over-expression | OS ↓ | 53 | CRC | Kollmann |
| PD-L1 | Over-expression | OS ↓ | 26 | Head and neck squamous cell cancer | Okada |
| PGE2 | Over-expression | Recurrence↓ | 53 | CRC | Lang |
| pSTAT3 | Evidence of nuclear staining in >15% of cancer cells | Recurrence, OS ↓ | 51 | CRC | Schweiger |
| VEGF-A | Staining area of ≥0.56 | OS ↓ | 29 | Osteosarcoma | Matsumoto |
| VEGF-C | Positive |
OS ↓ | 29 | Osteosarcoma | Matsumoto |
↓, decreased; ↑, increased. c-MET, tyrosine-protein kinase Met; CD8+ cells in TLS, CD8+ cytotoxic T-lymphocytes in tertiary-lymphoid structures; Ers, estrogen receptors; PRs, progesterone receptors; FOXP3+ TILs, FOXP3+ regulatory tumor infiltrating lymphocytes; Hsp27, Heat shock protein 27; KRAS, Kirsten rat sarcoma viral oncogene homologue; EGFR, epidermal growth factor; GNAQ, gene coding for the guanine nucleotide-binding protein Gq subunit alpha; KIT, gene coding for the tyrosine kinase c-KIT; MET, gene coding for c-MET; PTPN11, gene coding for the tyrosine-protein phosphatase non-receptor type 11; PDGFRA, platelet-derived growth factor receptor A; SMARCB1, SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily B member 1; NRAS, neuroblastoma RAS viral oncogene homolog; TP53, tumor protein p53; PD-1, programmed cell death protein-1; PD-L1, programmed cell death 1 ligand-1; PGE2, prostaglandin E2; pSTAT3, signal transducer and activator of transcription 3; VEGF-A, vascular endothelial growth factor A; VEGF-C, vascular endothelial growth factor C. *, coincident mutation detected by targeted next-generation sequencing.
c-MET and STAT3
The transmembrane tyrosine-protein kinase receptor Met (c-MET) and its downstream signaling mediator called signal transducer and activator of transcription 3 (STAT3) have been shown to play a key-role in cancer (71). In a cohort of 51 analyzed mCRC patients, immunohistochemical overexpression of c-MET and STAT3 was associated with decreased median OS compared to patients with low-expression (30 vs. 65 months, 24 vs. 65 months, respectively) (58). Furthermore, overexpression of STAT3—but not c-MET—was associated with early tumor recurrence after PM (10 vs. 19 months).
KRAS
The rate of KRAS mutations in patients with lung metastasis is 46%, which exceeds the rate of KRAS mutations in the overall cohort of patients with CRC. Furthermore, patients with KRAS mutation suffered from early recurrence of lung metastasis after PM (63). Renaud et al. evaluated and compared the impact of different KRAS mutation subtypes on clinical outcome. Especially mutations in exon 2 and codon 13 resulted in early pulmonary recurrence (56 vs. 78 months) and poor OS (54 vs. 82 months) after PM (64). Furthermore, it could be shown that patients with KRAS exon 2 codon 12 mutations seem to be good candidates for perioperative bevacizumab treatment. In the presence of codon 12 mutation, OS (101 vs. 55 months) as well as time to recurrence (70 vs. 24 months) were significantly higher when bevacizumab was part of the treatment regimen (72).
Tumor infiltrating lymphocytes (TILs) and tertiary lymphoid structures (TLS)
Subsets of TILs and so-called TLS are frequently found in the microenvironment as well as the stroma of numerous human cancers. TILs and TLS reflect an active immune response of the host against the tumor and seem to affect clinical outcome and survival (73,74). In a cohort of 57 CRC patients undergoing resection of pulmonary nodules, the density and distribution of TILs and TLS was investigated (59). High presence of FOXP3+ regulatory T-lymphocytes—which are known to have immune modulatory function—at the invasive front of the tumor was associated with decreased OS after PM (35 vs. 65 months). Moreover, low numbers of CD8+ cytotoxic T-lymphocytes—which are known for their anti-tumor effect—in surrounding TLS correlated with impaired OS (median survival not reached vs. 35 months).
Programmed cell death protein-1 (PD-1) and programmed cell death 1 ligand-1 (PD-L1)
PD-1 and its ligand PD-L1 are profoundly studied immune checkpoints and play a crucial role in suppressing endogenous activity of lymphocytes. Tumors try to escape immune surveillance by up-regulation and overexpression PD-1 and PD-L1. This mechanism is currently used as a target for specific antibodies blocking this pathway and thus supporting the anti-tumor effects of endogenous lymphocytes. Recently, it could be shown that PD-1 and PD-L1 are highly present in TILs of mCRC pulmonary metastasis specimen (68). Furthermore, overexpression of PD-1 by TILs was associated with poor OS (35 vs. 78 months) after PM. Another study presented by Okada et al. investigated 26 patients undergoing PM from primary head and neck squamous cell carcinoma. The median 5-year OS for patients with high PD-L1 expression was 16.7%, compared to 72.5% in case of low PD-L1 expression (69). Moreover, high PD-L1 expression was an independent prognostic factor for OS in multivariate analysis.
TP53
One of the most commonly studied tumor-suppressor genes is the TP53, coding for the protein p53 that plays an essential role cell cycle regulation and apoptosis of damaged cells. However, studies investigating the impact of p53 expression on outcome after PM failed to find a correlation (75,76). In a recent study by Li et al., overexpression of p53 was associated with impaired OS (46.1 vs. 62.6 months) however, level of significance was lost in multivariate analysis (67).
Comprehensive mutational profiles
Targeted next generation sequencing (tNGS) has become a routinely used tool of predictive molecular pathology in various epithelial entities. In 2016, Kovaleva and colleagues reported on various gene mutations found by tNGS in formalin-fixed and paraffin embedded CRC and lung metastasis specimen (77). Moreover, our group retrospectively analyzed the mutational profiles of 47 CRC patients undergoing PM using tNGS in primary tumor and pulmonary metastasis specimens (65). Cox regression model identified simultaneous mutation of TP53, SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily B member 1 (SMARCB1) and platelet-derived growth factor receptor A (PDGFRA) as being prognostic for poor OS after PM. Moreover, a coincident mutation of EGFR (gene coding for the epidermal growth factor), GNAQ (gene coding for the guanine nucleotide-binding protein Gq subunit alpha), KIT (a gene coding for the tyrosine kinase c-KIT), MET (a gene coding for the tyrosine kinase c-MET) and PTPN11 (a gene coding for the tyrosine-protein phosphatase non-receptor type 11) predicted early tumor recurrence after PM.
Prostaglandin-E2 (PGE2)
PGE2 is an inflammatory mediator, which regulates physiological—e.g., vasodilatation or muscle relaxation—but also pathophysiological—e.g., inflammation—processes. An overexpression of PGE2 has been reported in various malignancies (19), whereas its prognostic value remains elusive. In a cohort of 53 CRC patients undergoing PM, PGE2 was evident in 100% of the investigated metastasis specimen (70). Moreover, overexpression of PGE2 correlated with a significantly favorable tumor recurrence after PM (17 vs. 13 months). However, in multivariate analysis PGE2 could not be confirmed as a prognostic factor.
Estrogen receptors (ERs) and progesterone receptors (PRs)
The presence and therapeutic implication of steroid hormone receptors in human breast cancer had been recognized over 40 years ago (78). It has since become clear that the course of human breast cancer is dependent upon estrogen and/or progesterone triggered signals, mediated through ERs and PRs. Meimarakis and colleagues investigated 81 patients could show that the presence of ERs and/or PRs in pulmonary metastasis tissue from breast cancer significantly correlated with improved OS (127 vs. 27 months) after PM (60).
NRAS
The neuroblastoma RAS viral oncogene homolog
Heat-shock protein 27
Heat shock proteins represent a family of highly conserved proteins induced in response to various physiological and environmental insults, helping cells to survive. Among all heat shock proteins, Hsp27, Hsp7 and Hsp 90 are the most commonly studied subtypes. During physiological conditions—e.g., wound healing or keloid formation— activated fibroblasts express heat-shock protein 27 (Hsp27), which plays a crucial role for fibroblast motility and angiogenesis. However, expression of Hsp27 was reported to be high in cancer tissue and to strongly correlate with outcome (80,81). Addressing the prognostic value in PM, our group has previously investigated metastasis specimens of CRC patients and found high expression of Hsp27 in cancer-associated stroma of lung metastases (61). Moreover, extensive stromal Hsp27 expression was associated with decreased OS (31 vs. 52 months) and early tumor recurrence (10 vs. 19 months) after PM.
VEGF-A and VEGF-C
Subtypes of the vascular endothelial growth factor (VEGF) are well-known mediators in cancer-associated angiogenesis (82). However, one recent work investigated the prognostic value of two VEGF-subtypes on clinical outcome in context with PM (62). Patients with a low VEGF-A expression (defined as positive staining area <56%) and a negative VEGF-C staining in metastatic lesions from primary osteosarcoma showed significantly better OS in multivariate analysis (hazard ratio: 0.162, 95% confidence interval: 0.045–0.576 and hazard ratio: 0.058, 95% confidence interval: 0.008–0.421, respectively).
Ki67
Ki-67 is an established marker for determining the proliferation activity of normal and cancer cells. It has been shown that expression of Ki-67 is significantly higher in more aggressive and poorly differentiated tumor types. Different expression levels of Ki-67 strongly correlate with outcome in various cancer types, and moreover, there is increasing evidence that it may be an effective target for future cancer therapies (83). In context of PM, Matsumoto et al. investigated 29 osteosarcoma patients undergoing resection of pulmonary metastasis and found significant association between Ki-67 expression and long-term survival (62). Particularly, patients with high expression (≥0.33 positive staining for Ki67) had a significantly poorer OS (<60 months), compared to patients without expression of Ki67 in their pulmonary specimen (163 months).
Conclusions
Despite encouraging long-term survival rates after PM, there is a wide-variation of outcomes in terms of recurrence and remission (84). It seems that selection criteria—based on clinical factors—fail to differentiate between patients who benefit from a surgical resection and patients with rapid progression of disease. Several prognostic biomarkers have been investigated over the last decades and current available evidence was summarized in this review. Knowledge on the impact of these factors might help for the decision-making progress when selecting candidates for PM and for the development of future treatment strategies.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Michel Gonzalez) for the series “Advancement in the Surgical Treatment of Pulmonary Metastasis” published in Journal of Visualized Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jovs.2019.03.20). The series “Advancement in the Surgical Treatment of Pulmonary Metastasis” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ciombor KK, Wu C, Goldberg RM. Recent therapeutic advances in the treatment of colorectal cancer. Annu Rev Med 2015;66:83-95. [Crossref] [PubMed]
- Lee YH, Kang KM, Choi HS, et al. Comparison of stereotactic body radiotherapy versus metastasectomy outcomes in patients with pulmonary metastases. Thorac Cancer 2018;9:1671-9. [Crossref] [PubMed]
- Adachi M, Mizuno M, Mitsui H, et al. The prognostic impact of pulmonary metastasectomy in recurrent gynecologic cancers: a retrospective single-institution study. Nagoya J Med Sci 2015;77:363-72. [PubMed]
- Wiegering A, Riegel J, Wagner J, et al. The impact of pulmonary metastasectomy in patients with previously resected colorectal cancer liver metastases. PLoS One 2017;12:e0173933 [Crossref] [PubMed]
- Erhunmwunsee L, Tong BC. Preoperative Evaluation and Indications for Pulmonary Metastasectomy. Thorac Surg Clin 2016;26:7-12. [Crossref] [PubMed]
- Nakajima J, Iida T, Okumura S, et al. Recent improvement of survival prognosis after pulmonary metastasectomy and advanced chemotherapy for patients with colorectal cancer. Eur J Cardiothorac Surg 2017;51:869-73. [Crossref] [PubMed]
- Zabaleta J, Iida T, Falcoz PE, et al. Individual data meta-analysis for the study of survival after pulmonary metastasectomy in colorectal cancer patients: A history of resected liver metastases worsens the prognosis. Eur J Surg Oncol 2018;44:1006-12. [Crossref] [PubMed]
- Wang C, Yang L, Liang Z, et al. Long-Term Survival and Prognostic Factors of Pulmonary Metastasectomy in Liver Cancer: A Systematic Review and Meta-Analysis. World J Surg 2018;42:2153-63. [Crossref] [PubMed]
- Goossens N, Nakagawa S, Sun X, et al. Cancer biomarker discovery and validation. Transl Cancer Res 2015;4:256-69. [PubMed]
- Internullo E, Cassivi SD, Van Raemdonck D, et al. Pulmonary metastasectomy: a survey of current practice amongst members of the European Society of Thoracic Surgeons. J Thorac Oncol 2008;3:1257-66. [Crossref] [PubMed]
- Caristo JM, Tian DH, Yan TD. Pulmonary metastasectomy: a cross sectional survey. J Thorac Dis 2018;10:3757-66. [Crossref] [PubMed]
- Schweiger T, Lang G, Klepetko W, et al. Prognostic factors in pulmonary metastasectomy: spotlight on molecular and radiological markers. Eur J Cardiothorac Surg 2014;45:408-16. [Crossref] [PubMed]
- Renaud S, Romain B, Falcoz PE, et al. KRAS and BRAF mutations are prognostic biomarkers in patients undergoing lung metastasectomy of colorectal cancer. Br J Cancer 2015;112:720-8. [Crossref] [PubMed]
- Schweiger T, Kollmann D, Nikolowsky C, et al. Carbonic anhydrase IX is associated with early pulmonary spreading of primary colorectal carcinoma and tobacco smoking. Eur J Cardiothorac Surg 2014;46:92-9. [Crossref] [PubMed]
- Pereira AA, Rego JF, Morris V, et al. Association between KRAS mutation and lung metastasis in advanced colorectal cancer. Br J Cancer 2015;112:424-8. [Crossref] [PubMed]
- Ghidini M, Personeni N, Bozzarelli S, et al. KRAS mutation in lung metastases from colorectal cancer: prognostic implications. Cancer Med 2016;5:256-64. [Crossref] [PubMed]
- Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell 2011;147:275-92. [Crossref] [PubMed]
- Malumbres M, Barbacid M. RAS oncogenes: the first 30 years. Nat Rev Cancer 2003;3:459-65. [Crossref] [PubMed]
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature 2002;417:949-54. [Crossref] [PubMed]
- Dankner M, Rose AAN, Rajkumar S, et al. Classifying BRAF alterations in cancer: new rational therapeutic strategies for actionable mutations. Oncogene 2018;37:3183-99. [Crossref] [PubMed]
- Birner P, Jesch B, Friedrich J, et al. Carbonic anhydrase IX overexpression is associated with diminished prognosis in esophageal cancer and correlates with Her-2 expression. Ann Surg Oncol 2011;18:3330-7. [Crossref] [PubMed]
- Ilie M, Mazure NM, Hofman V, et al. High levels of carbonic anhydrase IX in tumour tissue and plasma are biomarkers of poor prognostic in patients with non-small cell lung cancer. Br J Cancer 2010;102:1627-35. [Crossref] [PubMed]
- Korkeila E, Talvinen K, Jaakkola PM, et al. Expression of carbonic anhydrase IX suggests poor outcome in rectal cancer. Br J Cancer 2009;100:874-80. [Crossref] [PubMed]
- van Kuijk SJ, Yaromina A, Houben R, et al. Prognostic Significance of Carbonic Anhydrase IX Expression in Cancer Patients: A Meta-Analysis. Front Oncol 2016;6:69. [Crossref] [PubMed]
- Wood KW, Sarnecki C, Roberts TM, et al. ras mediates nerve growth factor receptor modulation of three signal-transducing protein kinases: MAP kinase, Raf-1, and RSK. Cell 1992;68:1041-50. [Crossref] [PubMed]
- Yamamoto H, Itoh F, Senota A, et al. Expression of matrix metalloproteinase matrilysin (MMP-7) was induced by activated Ki-ras via AP-1 activation in SW1417 colon cancer cells. J Clin Lab Anal 1995;9:297-301. [Crossref] [PubMed]
- Jinesh GG, Sambandam V, Vijayaraghavan S, et al. Molecular genetics and cellular events of K-Ras-driven tumorigenesis. Oncogene 2018;37:839. [Crossref] [PubMed]
- Cho S, Song IH, Yang HC, et al. Prognostic factors of pulmonary metastasis from colorectal carcinoma. Interact Cardiovasc Thorac Surg 2013;17:303-7. [Crossref] [PubMed]
- Embun R, Rivas de Andres JJ, Call S, et al. Causal Model of Survival After Pulmonary Metastasectomy of Colorectal Cancer: A Nationwide Prospective Registry. Ann Thorac Surg 2016;101:1883-90. [Crossref] [PubMed]
- Sun F, Chen L, Shi M, et al. Prognosis of video-assisted thoracoscopic pulmonary metastasectomy in patients with colorectal cancer lung metastases: an analysis of 154 cases. Int J Colorectal Dis 2017;32:897-905. [Crossref] [PubMed]
- Hachimaru A, Maeda R, Suda T, et al. Repeat pulmonary resection for recurrent lung metastases from colorectal cancer: an analysis of prognostic factors. Interact Cardiovasc Thorac Surg 2016;22:826-30. [Crossref] [PubMed]
- Suzuki H, Kiyoshima M, Kitahara M, et al. Long-term outcomes after surgical resection of pulmonary metastases from colorectal cancer. Ann Thorac Surg 2015;99:435-40. [Crossref] [PubMed]
- Gonzalez M, Poncet A, Combescure C, et al. Risk factors for survival after lung metastasectomy in colorectal cancer patients: a systematic review and meta-analysis. Ann Surg Oncol 2013;20:572-9. [Crossref] [PubMed]
- Yokoyama S, Mitsuoka M, Kinugasa T, et al. Survival after initial lung metastasectomy for metastatic colorectal cancer in the modern chemotherapeutic era. BMC Surg 2017;17:54. [Crossref] [PubMed]
- Okumura T, Boku N, Hishida T, et al. Surgical Outcome and Prognostic Stratification for Pulmonary Metastasis From Colorectal Cancer. Ann Thorac Surg 2017;104:979-87. [Crossref] [PubMed]
- Osoegawa A, Kometani T, Fukuyama S, et al. Prognostic Factors for Survival after Resection of Pulmonary Metastases from Colorectal Carcinoma. Ann Thorac Cardiovasc Surg 2016;22:6-11. [Crossref] [PubMed]
- Hashimoto M, Tanaka F, Yoneda K, et al. The clinical value of circulating tumour cells (CTCs) in patients undergoing pulmonary metastasectomy for metastatic colorectal cancer. J Thorac Dis 2018;10:1569-77. [Crossref] [PubMed]
- Le UT, Bronsert P, Picardo F, et al. Intraoperative detection of circulating tumor cells in pulmonary venous blood during metastasectomy for colorectal lung metastases. Sci Rep 2018;8:8751. [Crossref] [PubMed]
- Ghanim B, Schweiger T, Jedamzik J, et al. Elevated inflammatory parameters and inflammation scores are associated with poor prognosis in patients undergoing pulmonary metastasectomy for colorectal cancer. Interact Cardiovasc Thorac Surg 2015;21:616-23. [Crossref] [PubMed]
- Li C, Xu Q, Chen L, et al. C-reactive protein (CRP) as a prognostic factor for colorectal cancer after surgical resection of pulmonary metastases. Bull Cancer 2017;104:232-6. [Crossref] [PubMed]
- Nakagawa T, Taguchi S, Kanatani A, et al. Oncologic Outcome of Metastasectomy for Urothelial Carcinoma: Who Is the Best Candidate? Ann Surg Oncol 2017;24:2794-800. [Crossref] [PubMed]
- Pastorino U, Morelli D, Leuzzi G, et al. Baseline and Postoperative C-reactive Protein Levels Predict Long-Term Survival After Lung Metastasectomy. Ann Surg Oncol 2019;26:869-75. [Crossref] [PubMed]
- Vodicka J, Spidlen V, Treska V, et al. Surgical treatment of colorectal cancer pulmonary metastases: 12-year results. Anticancer Res 2014;34:4239-45. [PubMed]
- Kobayashi S, Karube Y, Nishihira M, et al. Usefulness of Inflammation-Based Prognostic Score in Patients Undergoing Lung Metastasectomy for Colorectal Carcinoma. World J Surg 2016;40:1632-7. [Crossref] [PubMed]
- Renaud S, St-Pierre D, Spicer J, et al. Prognostic value of neutrophil to lymphocyte ratio in lung metastasectomy for colorectal cancer. Eur J Cardiothorac Surg 2018; [Epub ahead of print]. [Crossref] [PubMed]
- Thomsen M, Skovlund E, Sorbye H, et al. Prognostic role of carcinoembryonic antigen and carbohydrate antigen 19-9 in metastatic colorectal cancer: a BRAF-mutant subset with high CA 19-9 level and poor outcome. Br J Cancer 2018;118:1609-16. [Crossref] [PubMed]
- Grunnet M, Sorensen JB. Carcinoembryonic antigen (CEA) as tumor marker in lung cancer. Lung Cancer 2012;76:138-43. [Crossref] [PubMed]
- Meng Q, Shi S, Liang C, et al. Diagnostic and prognostic value of carcinoembryonic antigen in pancreatic cancer: a systematic review and meta-analysis. Onco Targets Ther 2017;10:4591-8. [Crossref] [PubMed]
- Gkountela S, Szczerba B, Donato C, et al. Recent advances in the biology of human circulating tumour cells and metastasis. ESMO Open 2016;1:e000078 [Crossref] [PubMed]
- Kaifi JT, Li G, Clawson G, et al. Perioperative circulating tumor cell detection: Current perspectives. Cancer Biol Ther 2016;17:859-69. [Crossref] [PubMed]
- Ghanim B, Hoda MA, Winter MP, et al. Pretreatment serum C-reactive protein levels predict benefit from multimodality treatment including radical surgery in malignant pleural mesothelioma: a retrospective multicenter analysis. Ann Surg 2012;256:357-62. [Crossref] [PubMed]
- O'Dowd C, McRae LA, McMillan DC, et al. Elevated preoperative C-reactive protein predicts poor cancer specific survival in patients undergoing resection for non-small cell lung cancer. J Thorac Oncol 2010;5:988-92. [Crossref] [PubMed]
- McMillan DC. The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev 2013;39:534-40. [Crossref] [PubMed]
- Erpenbeck L, Schön MP. Neutrophil extracellular traps: protagonists of cancer progression? Oncogene 2017;36:2483. [Crossref] [PubMed]
- Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860-7. [Crossref] [PubMed]
- Guthrie GJ, Charles KA, Roxburgh CS, et al. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol 2013;88:218-30. [Crossref] [PubMed]
- Ballehaninna UK, Chamberlain RS. The clinical utility of serum CA 19-9 in the diagnosis, prognosis and management of pancreatic adenocarcinoma: An evidence based appraisal. J Gastrointest Oncol 2012;3:105-19. [PubMed]
- Schweiger T, Starkl V, Glueck O, et al. Clinical impact of c-MET expression and mutational status in patients with colorectal cancer lung metastases. Eur J Cardiothorac Surg 2016;49:1103-11; discussion 1111. [Crossref] [PubMed]
- Schweiger T, Berghoff AS, Glogner C, et al. Tumor-infiltrating lymphocyte subsets and tertiary lymphoid structures in pulmonary metastases from colorectal cancer. Clin Exp Metastasis 2016;33:727-39. [Crossref] [PubMed]
- Meimarakis G, Rüttinger D, Stemmler J, et al. Prolonged Overall Survival After Pulmonary Metastasectomy in Patients With Breast Cancer. Ann Thorac Surg 2013;95:1170-80. [Crossref] [PubMed]
- Schweiger T, Nikolowsky C, Starlinger P, et al. Stromal expression of heat-shock protein 27 is associated with worse clinical outcome in patients with colorectal cancer lung metastases. PLoS One 2015;10:e0120724 [Crossref] [PubMed]
- Matsumoto I, Oda M, Yachi T, et al. Outcome Prediction of Pulmonary Metastasectomy Can Be Evaluated Using Metastatic Lesion in Osteosarcoma Patients. World J Surg 2013;37:1973-80. [Crossref] [PubMed]
- Schweiger T, Hegedus B, Nikolowsky C, et al. EGFR, BRAF and KRAS status in patients undergoing pulmonary metastasectomy from primary colorectal carcinoma: a prospective follow-up study. Ann Surg Oncol 2014;21:946-54. [Crossref] [PubMed]
- Renaud S, Guerrera F, Seitlinger J, et al. KRAS exon 2 codon 13 mutation is associated with a better prognosis than codon 12 mutation following lung metastasectomy in colorectal cancer. Oncotarget 2017;8:2514-24. [Crossref] [PubMed]
- Schweiger T, Liebmann-Reindl S, Glueck O, et al. Mutational profile of colorectal cancer lung metastases and paired primary tumors by targeted next generation sequencing: implications on clinical outcome after surgery. J Thorac Dis 2018;10:6147-57. [Crossref] [PubMed]
- Ulivieri A, Cardillo G, Manente L, et al. Molecular characterization of a selected cohort of patients affected by pulmonary metastases of malignant melanoma: Hints from BRAF, NRAS and EGFR evaluation. Oncotarget 2015;6:19868-79. [Crossref] [PubMed]
- Li C, Xu Q, Chen L, et al. Prognostic value of p53 for colorectal cancer after surgical resection of pulmonary metastases. World J Surg Oncol 2016;14:308. [Crossref] [PubMed]
- Kollmann D, Schweiger T, Schwarz S, et al. PD1-positive tumor-infiltrating lymphocytes are associated with poor clinical outcome after pulmonary metastasectomy for colorectal cancer. Oncoimmunology 2017;6:e1331194 [Crossref] [PubMed]
- Okada S, Itoh K, Ishihara S, et al. Significance of PD-L1 expression in pulmonary metastases from head and neck squamous cell carcinoma. Surg Oncol 2018;27:259-65. [Crossref] [PubMed]
- Lang C, Hrdliczka E, Schweiger T, et al. Impact of cyclooxygenase-2 and prostaglandin-E2 expression on clinical outcome after pulmonary metastasectomy. J Thorac Dis 2017;9:621-35. [Crossref] [PubMed]
- Organ SL, Tsao MS. An overview of the c-MET signaling pathway. Ther Adv Med Oncol 2011;3:S7-S19. [Crossref] [PubMed]
- Renaud S, Schaeffer M, Falcoz PE, et al. Perioperative bevacizumab improves survival following lung metastasectomy for colorectal cancer in patients harbouring v-Ki-ras2 Kirsten rat sarcoma viral oncogene homologue exon 2 codon 12 mutationsdagger. Eur J Cardiothorac Surg 2017;51:255-62. [PubMed]
- Gooden MJ, de Bock GH, Leffers N, et al. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer 2011;105:93-103. [Crossref] [PubMed]
- Colbeck EJ, Ager A, Gallimore A, et al. Tertiary Lymphoid Structures in Cancer: Drivers of Antitumor Immunity, Immunosuppression, or Bystander Sentinels in Disease? Front Immunol 2017;8:1830. [Crossref] [PubMed]
- Shiono S, Ishii G, Nagai K, et al. Immunohistochemical prognostic factors in resected colorectal lung metastases using tissue microarray analysis. Eur J Surg Oncol 2006;32:308-9. [Crossref] [PubMed]
- Kayser K, Zink S, Andre S, et al. Primary colorectal carcinomas and their intrapulmonary metastases: clinical, glyco-, immuno- and lectin histochemical, nuclear and syntactic structure analysis with emphasis on correlation with period of occurrence of metastases and survival. Apmis 2002;110:435-46. [Crossref] [PubMed]
- Kovaleva V, Geissler AL, Lutz L, et al. Spatio-temporal mutation profiles of case-matched colorectal carcinomas and their metastases reveal unique de novo mutations in metachronous lung metastases by targeted next generation sequencing. Mol Cancer 2016;15:63. [Crossref] [PubMed]
- McGuire WL, Horwitz KB, Pearson OH, et al. Current status of estrogen and progesterone receptors in breast cancer. Cancer 1977;39:2934-47. [Crossref] [PubMed]
- Bos JL. ras oncogenes in human cancer: a review. Cancer Res 1989;49:4682-9. [PubMed]
- Wang X, Chen M, Zhou J, et al. HSP27, 70 and 90, anti-apoptotic proteins, in clinical cancer therapy Int J Oncol 2014;45:18-30. (Review). [Crossref] [PubMed]
- Vidyasagar A, Wilson NA, Djamali A. Heat shock protein 27 (HSP27): biomarker of disease and therapeutic target. Fibrogenesis Tissue Repair 2012;5:7. [Crossref] [PubMed]
- Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology 2005;69:4-10. [Crossref] [PubMed]
- Li LT, Jiang G, Chen Q, et al. Ki67 is a promising molecular target in the diagnosis of cancer Mol Med Rep 2015;11:1566-72. (review). [Crossref] [PubMed]
- Petrella F, Diotti C, Rimessi A, et al. Pulmonary metastasectomy: an overview. J Thorac Dis 2017;9:S1291-S1298. [Crossref] [PubMed]
Cite this article as: Lang C, Hoetzenecker K, Schweiger TS. Emerging biomarkers in pulmonary metastasectomy. J Vis Surg 2019;5:44.